Comparative Toxicity of Endosulfan and Fipronil Insecticides: Utilizing In Vivo and In Vitro Data
Marilyn H Silva and Svetlana Koshlukova
DOI10.21767/2471-9641.10009
Department of Pesticide Regulation, California Environmental Protection Agency, Sacramento, CA, USA 95812, USA
- Corresponding Author:
- Marilyn H Silva
Department of Pesticide Regulation
California Environmental Protection Agency
Sacramento, CA, USA 95812
Tel: 916 324 3482
E-mail: Marilyn.silva@cdpr.ca.gov
Received date: October 09, 2015, Accepted date: October 31, 2015, Published date: November 07, 2015
Citation: Silva MH, Koshlukova S. Comparative Toxicity of Endosulfan and Fipronil Insecticides: Utilizing In Vivo and In Vitro Data. J Med toxicol clin forensic med. 2016, 1:1.
Abstract
Background: Endosulfan, an organochlorine compound, and fipronil, a phenylpyrazole, are insecticides with a common mechanism of toxicity. They interfere with Cl- influx by binding to the gamma-aminobutyric acid receptor (GABAAR) and blocking the inhibitory actions of GABAA. In vivo they cause neurotoxicity, hepatotoxicity, developmental toxicity, and can alter endocrine and immune systems. The thyroid is a target of fipronil toxicity. Human exposure occurs via food residues, skin contact and/or air dispersion. They are environmentally persistent and bioaccumulate in food chains.
Method: Compare in vivo data with in vitro results from the U.S. Environmental Protection Agency (USEPA) Toxicology Forecaster (ToxCast) high-throughput screening assays and zebrafish models to assess their usefulness in predicting toxicity.
Results: Fipronil’s in vivo toxicity occurred at lower doses than endosulfan for similar effects. ToxCast was a weak predictor of liver toxicity and estrogen receptor interaction. Missing is evidence of “true actives” for fipronil ToxCast assays with the thyroid receptor and for either compound with GABAAR or androgen receptors. Zebrafish models were good predictors of endosulfan and fipronil neurotoxicity in mammalian in vivo studies. Conclusion: ToxCast assays do not provide support for in vivo neurotoxicity or endocrine disruption where zebrafishs are good predictors of both parameters.
Key Words
Endosulfan; Fipronil; Organochlorine; GABA Inhibitors; Toxcast; Zebrafish; Carcinogens
Introduction
Endosulfan (ES: 6, 7, 8, 9, 10, 10-hexachloro-1, 5, 5α, 6, 9, 9α-hexahydro-6, 9-methano-2, 4, 3-benzodioxathiepin-3-oxide; [isomers: α-; β-]) and fipronil (FP: (RS)-5-amino-1-[2,6-dichloro-4- (trifluoromethyl)phenyl]-4- (trifluoromethylsulfinyl)-1H-pyrazole- 3-carbonitrile) represent the first and second generations, respectively, of chloride channel blocker insecticides whose primary target is the central nervous system [1,2]. High potential for human exposure is indicated for both pesticides. Exposure to general public results from ingesting residues in food, inhaling vapors, skin contact. Workers’ exposures are mainly dermal and inhalation from handling or re-entering treated fields.A large number of poisonings are reported from fipronil’s use in veterinary products for flea and tick control. The exposure is likely higher for general public living near hazardous waste sites where endosulfan has been detected [3,4]. As noncompetitive antagonists of the GABAA-receptor, they block passage of Clions and thereby the actions of the inhibitory neurotransmitter GABA [2]. In mammals, seizures, vomiting and convulsions are symptoms of excessive nerve stimulation associated with GABAA antagonism [5]. Their action in the brain is very complex due to biotransformation to toxic metabolites that can act on GABAAR (e.g., FP sulfone [6]). Each is detoxified by P450s (CYP2B6, CYP3A4-5: ES; hCYP2C9: FP) [7,8] but neither requires activation to be toxic8. ES and FP are associated with endocrine disruption. FP has induced thyroid and liver cancer in animal models (https://www.cdpr.ca.gov/ docs/risk/toxsums/toxsumlist.htm; accessed 9/2015) [9]. These well characterized in vivo effects provide a basis for comparing toxicities.
Another means of comparison is the U.S. Environmental Protection Agency (USEPA) Toxicology Forecaster (ToxCast) in vitro highthroughput screening program; profiling more than 1,000 chemicals (~ 800 assays https://actor.epa.gov/dashboard2/; accessed 9/2015). In addition, zebrafish models are a rapid in vivo method for assessing developmental effects [10-13]. The goal of this brief communication is to examine if ToxCast and zebrafish results were predictive of targets and activities relevant to in vivo ES and FP endpoints.
Method
in vivo studies
in vivo studies in Table 1 summarize relevant neurotoxic or endocrine disrupting effects for both chemicals. No-Observed- Effect-Levels (NOEL) and Lowest-Observed-Effect-Levels (LOEL) were established by the California Department of Pesticide Regulation from guideline studies required for pesticide registration (https://www.cdpr.ca.gov/docs/risk/rcd.htm) and from open literature.
In vitro studies
We accessed the recently updated ToxCast database (https://actor.epa.gov/dashboard2/) for active assays that could inform effects manifested in overt toxicity in in vivo studies.
Zebrafish studies
Data from two published methods included embryos treated with chorion intact [10,11] or with chorion removed [12,13].
in vivo to in vitro extrapolation (IVIVE)
IVIVE was used to convert zebrafish NOELs expressed as μM concentrations into oral equivalent doses (OED: mg/kg/d). Estimated pharmacokinetic data derived from linear regression, published in vitro hepatic metabolism and protein binding with rat data are used for the conversion [14].
Results
in vivo
Both insecticides were neurotoxic [15,16] with LOELs of 2-2.5 mg/kg/d in rats and dogs (Table 1). ES developmental effects in rat pups and fetuses occurred at up to 7-fold higher doses than FP. Potential endocrine disruption was shown in both compounds by developmental delays and skeletal variations. Liver effects in rats and mice at LOELs of 3.95 mg/kg/d (ES) and 0.13 mg/ kg/d (FP) included increased liver weights and hepatocellular carcinomas in mice (FP [17]). FP thyroid toxicity was evident by altered thyroid function and follicular cell adenomas in rats (LOEL 0.06 mg/kg/d) [18].
ToxCast data
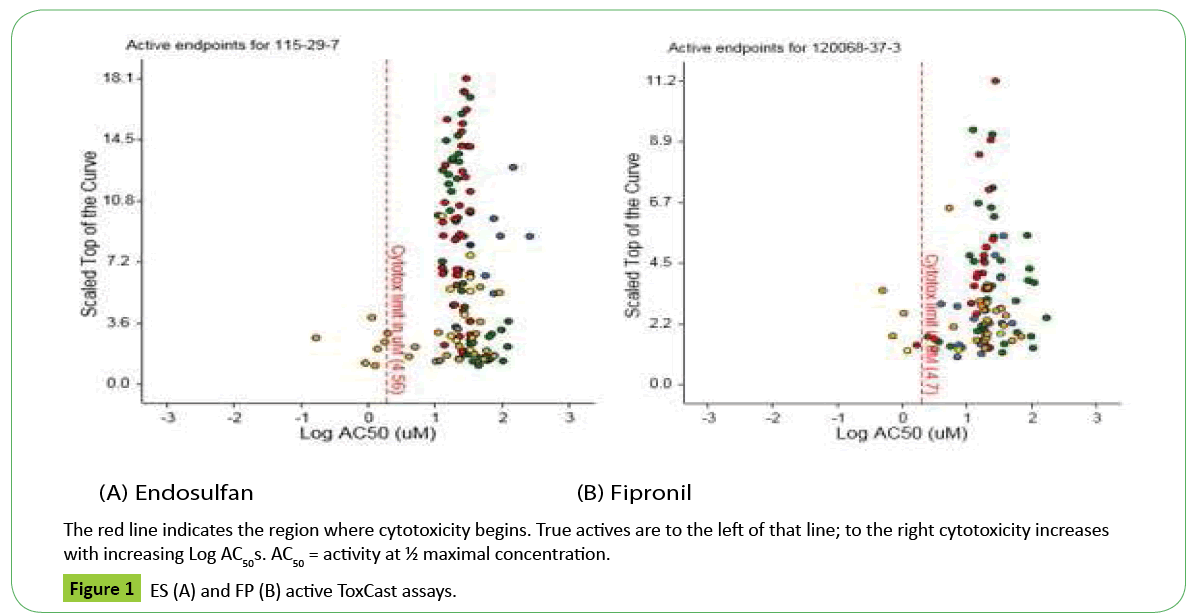
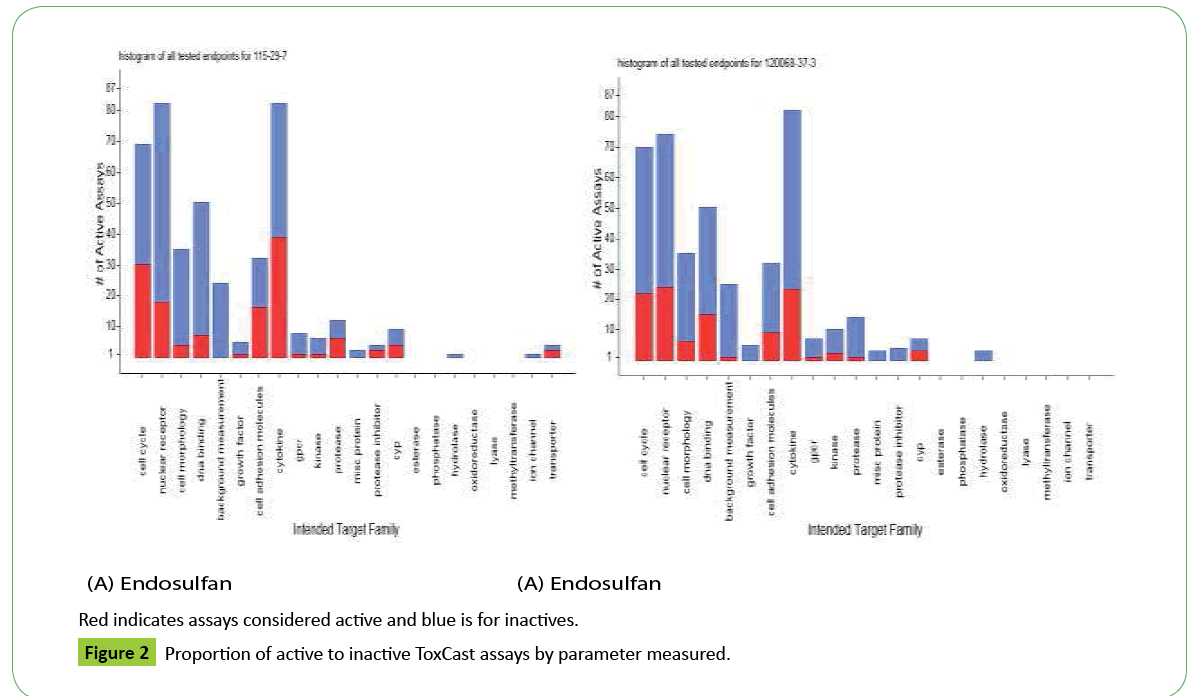
Results are reported as AC50s (½ maximal activity concentrations: Dashboard: https://actor.epa.gov/dashboard/2.All active assays appear in Figure 1 (dots) to the right and left of the cytotoxicity limit; however true actives have AC50s below the cytotoxicity limits (e.g. < 4.56 uM: ES; < 4.7 uM FP). Active assays beyond the cytotoxicity limit may not be specific to a chemical-receptor interaction but represent a burst of cellular responses indicative of cytotoxicity. ES true actives were assays having to do with xenobiotic metabolism (human constitutive androstane receptor: hCAR; immune response: DR5; pregnane-x: affecting hCYP2B6 genes; hCYP3A4 xenobiotic response element: PXRE) and the estrogen receptor (ERα; estrogen response element: ERE). True actives for FP were related to cell adhesion molecules (kinases), hCYP2C9, xenobiotic metabolism (hCAR) and ERE. Figure 2 shows active versus inactive assays and associated components for each compound (abbreviations at: https://www.epa.gov/comptox/ toxcast/data.html).
Zebrafish data
Zebrafish had an AC50 of 1 μM after exposure to ES (chorion intact) [10]; with a Toxicity Score (all malformations) of 40 (highest possible score) at peak concentrations (4 μM). When embryo chorions were removed, ES was highly toxic to larval development at > 0.0064 μM [12]. ESα-treated embryos (chorion intact) showed neurobehavioral effects (abnormal swim behavior, disorientation, abnormal touch response) at > 1.0 μM (NOEL 0.5 μM) [22].
FP-treated zebrafish (chorion intact) had an AC50 of 15.5 μM with a “Toxicity Score” of 40 [10]. Without the chorion, however, FP had no effects on development [10]. Zebrafish (chorion removed) treated with FP at > 0.23 μM had irreversible effects on body length, notochord degeneration, abnormal axial muscle morphology, muscle fiber degeneration [11].
IVIVE for ES neurotoxicity in zebrafish (chorion removed) [13] estimated an OED of 0.019 mg/kg/d (0.5 μM NOEL) [9]; 25-fold lower than the neurotoxicity NOEL in dogs (0.5 mg/kg/d) (Table 1) [15]. For FP the zebrafish OED was 0.37 mg/kg/d based on neurotoxicity at 23 μM [13]. This OED is equivalent to the in vivo NOEL (0.2 mg/kg/d) from an acute neurotoxicity rat study [16].
Discussion/Conclusion
Data indicate that in vivo, FP’s toxicity occurred at lower doses than ES (Table 1) for parameters examined. It is classified as a “possible human carcinogen” based on thyroid tumors in rat. ToxCast true actives for CYPs (ES: hCYP2B6 & hCYP3A4; FP: hCYP2C9) correlated well with in vivo toxicity [7, 8]. Other true activities for ES and FP were for generalized liver enzyme activity, which could be predictive of liver toxicity observed in vivo.
Missing were true actives for the GABAAR ToxCast assays (0/5 total) for either compound. Since neither compound needs metabolic activation to be toxic, the inactivity could be due to a lack of adequate assay design.
ES had 2/18 total true estrogen-receptor actives and FP had 1/18. There were no true actives for the androgen receptor (0/11 total) and (especially for FP) the thyroid receptor (0/4 total). There was activity for ER, AR and TR (https://actor.epa. gov/dashboard/2) but only above the cytotoxicity limit). There was poor correlation between in vivo endocrine disruption from both chemicals and true actives with ER, AR and TR in ToxCast.
Zebrafish assays were useful for predicting developmental neurotoxicity. The OEDs reasonably correlated with NOELs observed in vivo: (ES: 25 fold difference; FP equivalent). With the continued rapid development of the ToxCast and zebrafish assays, they could be used to support modes of action and adverse outcome pathways for new chemicals.
Disclaimer
The opinions and conclusions expressed in this paper are those of the authors and do not necessarily represent the views or opinions of the Department of Pesticide Regulation. The authors state that their design and interpretation is not compromised by any sponsor as a condition of review and publication.
References
- Skellern C (2015) Minimising bias in the forensic evaluation of suspicious paediatric injury. J Forensic Leg Med 34:11-16.
- Block R (2015) Minimising (sic) bias in the forensic evaluation of suspicious paediatric injury. The Quarterly Update 22: 15-16.
- Laskey AL (2014) Cognitive errors: thinking clearly when it could be child maltreatment. Pediatr Clin North Am 61: 997-1005.
- Committee on Identifying the Needs of the Forensic Sciences Community, National Research Council National Academy of Sciences (2009) Strengthening forensic science in the United States: a path forward. National Academies Press
- Dror IE, Hampikian G(2011) Subjectivity and bias in forensic DNA mixture interpretation. Science and Justice51: 204-208.
- Kassin SM, Dror IE, Kukucka J (2013) The forensic confirmation bias: problems, perspectives, and proposed solutions. J Appl Res Mem Cognition 2: 42-52.
- Freckelton I, Selby H (1999) The law of expert evidence. Sydney,LBC.
- Adams JA (2011) Medical evaluation of suspected child sexual abuse: 2011 update. J Child Sex Abuse20: 588-605.
- Jenny C, Hymel KP, Ritzen A, Reiner SE, Hay TC (1999) Analysis of missed cases of abusive head trauma.JAMA 281: 621-626.
- Pinto P S, Meoded A, Poretti A, Tekes A, Huisman T (2012) The Unique Features of Traumatic Brain Injury in Chidren, Review of the Characteristics of hte Peditric Skull and Brain, Mechanisms of Trauma, Patterns of Injury, Complications, and their Imaging Findings - Part 2. J Neuroimaging 22: e18-e41.
- Pinnock R, Welch P (2014) Learning clinical reasoning. J Paediatr Child Health 50: 253-257.
- Skellern C, Donald T (2012) Defining standards for medico-legal reports in forensic evaluation of suspicious childhood injury. J Forensic Leg Med 19: 267-271.
- Skellern C, Donald T (2014) The relevance of the Goudge inquiry to the practice of child protection/forensic paediatrics. J Forensic Leg Med 27: 35-38.
- Skellern C, Donald T (2011) Suspicious childhood injury: formulation of forensic opinion. J Paediatr Child Health 47: 771-775.
- Mitchell D, Eckstein D (2009) Jury dynamics and decision-making: a prescription for groupthink. Int J Acad Res 1: 163-169.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences