The Propolis Effect on Chlorpyrifos Induced Thyroid Toxicity in Male Albino Rats
Arwa A El-Sheikh and Hanaa M Ibrahim
DOI10.21767/2471-9641.100023
Arwa A El-Sheikh1 and Hanaa M Ibrahim2
1Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine,Zagazig University, Egypt
2Department of Pathology, Faculty of Medicine, Zagazig University, Egypt
- *Corresponding Author:
- Arwa A El-Sheikh
Department of Clinical Toxicology
Zagazig University, Egypt
Tel: 00201004169341
E-mail: sunsetaro@yahoo.com
Received date: December 10, 2016; Accepted date: February 01, 2017; Published date: February 08, 2017
Citation: El-Sheikh AA, Ibrahim HM. The Propolis Effect on Chlorpyrifos Induced Thyroid Toxicity in Male Albino Rats. J Med Toxicol Clin Forensic Med. 2017, 3:3.
Abstract
Chlorpyrifos (CPF) is a chlorinated organophosphate insecticide that is used widely in the world and Egypt. The aim of present work was to study the possible protective role of Propolis on thyroid gland of male rats after chronic exposure of chlorpyrifos. For this purpose, Fifty adult male albino rats were randomized into 5 groups: I, II (Control), III (received Propolis dissolved in 0.5 ml corn oil, 50 mg/kg b.w./day), IV (received CPF dissolved in 0.5 ml corn oil 6.75 mg/kg b.w./ day) and V (received CPF and Propolis at the same previously mentioned doses). Rats were orally treated by gavage for 12 weeks. We found that Chlorpyrifos induced damaging effects in thyroid tissue architecture, thickened collagen fibers between the follicles and decreased periodic acid schiff (PAS) reaction in the colloids. Immunohistochemically, there was weak thyroglobulin (TGB) expression. Besides, it significantly decreased the serum levels of Tri-iodothyronin (T3), Tetraiodothyronin (T4) and Thyroid stimulating hormone (TSH). It depressed reduced glutathione (GSH) content and antioxidant enzyme activities in thyroid tissues with respect to control groups. Furthermore, CPF increased thyroid oxidative stress as manifested by elevated malondialdehyde (MDA) levels. On the other hand, the concurrent administration of Propolis with CPF showed significant improvement in previous changes. Our findings showed that the concurrent oral administration of Propolis with CPF improved the CPF induced oxidative damaging effects in thyroid gland and the antioxidative defense in the rats.
https://myseoblog.blogdon.net/
https://myseoblog.blogaaja.fi/
https://myseoblog.jimdosite.com/
https://myseoblog.edublogs.org/
https://myseoblog.websites.co.in/
https://myseoblog47.wordpress.com/
https://myseoblog.waarnnnnnnbenjij.nu/
https://myseoblog.jigsy.com/
https://szeith-rhounds-kliagy.yolasite.com/
https://myseoblog-40.webselfsite.net/
https://myseoblog.mystrikingly.com/
https://myseoblog.splashthat.com/
https://myseoblog.webnode.com.tr/
https://myseoblog.odoo.com/
https://myseoblog.creatorlink.net/
https://whiteseotr1-s-site.thinkific.com/
https://myseoblog.estranky.cz/
https://65390c7d9a166.site123.me/
https://myblogseoooo.blogspot.com/
https://myseoblog.hashnode.dev/
https://whiteseotr1.wixsite.com/myseoblog
https://myseoblogg.weebly.com/
https://sites.google.com/view/myseoblogg/
https://codepen.io/myseoblog/pens/public
https://myseoblogg.livejournal.com/
https://wakelet.com/@myseoblog87204
https://www.homify.com/users/9537482/myseoblog/
https://theomnibuzz.com/author/myseoblog/
https://lessons.drawspace.com/profile/323508/myseoblog/
https://my.desktopnexus.com/myseoblog/
https://writeupcafe.com/profile/myseoblog/
https://www.pearltrees.com/myseoblog
https://www.easyfie.com/myseoblog
https://pharmahub.org/members/27544
https://www.zupyak.com/u/myseoblog/posts
https://www.metroflog.co/myseoblog
https://www.fuzia.com/fz/myseoblog-myseoblog
https://tr.pinterest.com/whiteseotr1/
https://my.getjealous.com/myseoblog
https://micro.blog/myseoblog
https://www.tumblr.com/blog/myseobloggsblog
https://hub.docker.com/u/myseoblog
https://fire.blogfree.net/?act=Profile&MID=1342100
https://myseoblog.pixnet.net/blog
https://myseoblogg.seesaa.net/
https://www.threadless.com/@myseoblog/activity
https://neocities.org/site/myseoblog
https://myseoblog.amebaownd.com/
https://teletype.in/@myseoblog
https://ubl.xml.org/users/myseoblog S6t3Bh9Gwo
https://educatorpages.com/site/myseoblog/
https://myseoblog.onlc.fr/
Keywords
Chlorpyrifos; Propolis; Thyroid hormones; Thyroglobulin; Oxidative stress; Antioxidant.
Introduction
Organophosphate insecticides (OPI) are used excessively in large areas causing environmental pollution, and therefore, are a cause of concern [1]. They are the commonest type of insecticides used worldwide and employed in domestic, agricultural and nonagricultural settings [2].
Chlorpyrifos (CPF) is an OPI that is used to control household, public health insects, mushroom, flies, aphids, spider mites, caterpillars, white flies in greenhouse, outdoor fruits and vegetable crops [3]. Chloropyrifos produces its toxic effects by acetyl cholinesterase (AChE) inhibition [4]. Furthermore, oxidative stress induced by OPI has been implicated in their toxicities [5]. The long-term exposure to CPF causes many chronic effects on different organs [6]. It is considered as a potential endocrine disrupter [7].
The thyroid gland is the most commonly affected endocrine gland as it is considered a sensitive target to OPI leading to affection of thyroid function [8]. Thyroid hormones are important in several physiological processes such as metabolism and normal growth development and any imbalance in their levels could lead to a wide range of clinical conditions [9].
Propolis is bee hive glue produced by honey bees and contains more than 160 constituents [10]. The most important active constituents in Propolis are flavonoids and various phenolic that are thought to be responsible for many effects as anticancer anti-inflammatory and antioxidant effects due to its ability to scavenge free radicals and protect lipids from being oxidized or destroyed during oxidative damage [11-14].
Therefore, the present work was carried out to study the toxic effect of chronic exposure to CPF on thyroid gland, to investigate the possible underlying mechanisms and to study the role of Propolis against these CPF toxic effects.
Materials and Methods
Chemicals
Chlorpyrifos (ISO common name E-ISO, BSI, ANSI, ESA) (O, O-diethyl-O-3, 5, 6-trichloro-2-pyridyl phosphorothioate) white powder; CAS NO: 285138-81-0; purity of 99% was purchased from Sigma, Aldrich, Germany and imported by Cairo Chemical Company, Egypt. It was dissolved in corn oil.
Propolis was obtained from beehives in Zagazig, Alsharqia governorate, Egypt in September 2013. The samples of Propolis were kept in an ice box at 4°C for one week then it was dehydrated and the dried Propoils was ground to fine brownish powder. The Ethanolic Propolis extract 80% was prepared as described by Park and Ikeigaki by adding 5 g of Propolis and 60 ml of 80% ethanol then shaking at 70°C until complete thawing [15]. The mixture was centrifuged and the supernatant was obtained and aqueous solution from distilled water of Propolis was performed.
Experimental animals
Fifty adult male albino rats were purchased from the animal house of the Faculty of Veterinary Medicine, Zagazig University weighting (180-200 g). Rats were acclimatized for 1 week before use in the experiment. They were kept in stainless steel cages under standardized environmental conditions (25°C). They were allowed for laboratory rat chow diet and water ad-libitum. Strict care and hygiene were taken to maintain a normal and healthy environment for all rats all time. All ethically approved conditions used for animal housing & handling were considered. All rats received human care in compliance with the guidelines of the Medical Research Ethics Committee of Zagazig University and met with those acquired by applicable international laws and regulations [16].
Experimental design
The rats were equally divided into five groups and caged separately. All treatments were given by oral gavage for 12 weeks.
Group I (Control I): (10 rats), the rats received 0.5 ml of distilled water once daily.
Group II (Control II): (10 rats), the rats received 0.5 ml of corn oil once daily.
Group III (Propolis group): (10 rats), the rats received Propolis dissolved in distilled water (50 mg/kg b.w./day). The dose was adjusted according to previous studies [17-19].
Group IV (CPF group): (10 rats), the rats received Chlorpyrifos dissolved in corn oil (6.75 mg/Kg b.w./day) [20, 21]. This dose represented 1/20 of oral LD50 (135 mg/kg) [22, 23].
Group V (CPF+Propolis group): (10 rats), the rats received CPF concurrently with Propolis in the same mentioned doses.
24 h after administration of the last dose, the rats had been fasted over-night and venous blood samples were collected from retroorbital plexus of each rat while the animal was anesthetized with ether and the serum was obtained from each blood sample by centrifugation at 3000 rpm for 10 min for evaluation of T3, T4 and TSH. The animals were then euthanized by cervical dislocation and the thyroid glands were excised. Each thyroid was dissected into two parts; one part was prepared for histopathological and immunohistochemical examination. The other part was isolated in ice cold media for tissues homogenates preparation for determination of MDA, GSH, GPX and SOD.
Hormonal assay
Hormonal analysis of Tri-iodothyronin (T3), Tetra-iodothyronin (T4) and Thyroid stimulating hormone (TSH) was performed by enzyme-linked immunosorbent assay (ELISA) using (Abnova mouse/rat T3 and the data expressed as ng/ml, Genway mouse/ rat T4 and the data expressed as ng/ml and Kamiya Biomedical Company rat TSH kits and the data expressed as pg/ml).
Oxidative and antioxidants assay
Thyroid gland tissues levels of MDA as a byproduct of lipid peroxidation, SOD, GSH and GPX as antioxidants markers were measured according to instructions of commercial kits of Egypt Biodiagnostic.
Malondialdehyde (MDA) was determined spectrophotometrically according to the data were expressed as nmol/g tissue processed [24].
Reduced glutathione (GSH) content was measured spectrophotometrically according to the method of the data were reported as mmol/g tissue processed [25].
Glutathione peroxidase (GPX) activity was determined spectrophotometrically according to the method of the GPX activity was expressed as U/g protein [26].
Superoxide dismutase (SOD) activity was assayed according to the method of the data were expressed as U/g protein [27].
Histopathological evaluation of thyroid gland
Histological study
Thyroid gland sections of rats were fixed in 10% neutral buffered formalin (pH 7.2), dehydrated in ascending series of ethanol, cleared in methyl benzoate, embedded in paraffin wax, deparaffinized with xylene and 4-5 μm thick sections blocks were prepared and stained with Haematoxylin and Eosin stain (H&E) for gross histological evaluation and Masson’s trichrome stain to differentiate the collagen Fibers where the nuclei were stained with black, the cytoplasm was stained with red and the collagen was blue [28-30].
Histochemical study
Five μm thick sections blocks were prepared and stained with Periodic acid–Schiff (PAS) reaction to detect the changes of the colloid where PAS positive materials were stained magenta and the nuclei were blue [31, 32].
Immunnohistochemical evaluation of thyroid gland
Immunostaining for thyroglobulin (TGB) was performed using mouse monoclonal antibody (kit from Thermo Scentific/Lab Vision Corporation, Fermont, USA and clone: TGB04+TGB05. Dilution 1:100) according to the avidin-biotin-peroxidase complex (ABC) method. Formalin fixed paraffin tissues were cut into 4-μm thick sections and transferred to positively charged slides. Then, sections were subjected to de-waxing, rehydration, blocking with hydrogen peroxide and antigen retrieval with microwave. The slides were then incubated overnight at 2-8°C with the primary antibody. Incubation with secondary antibody and product visualization was performed with diaminobenzidine substrate as the chromogen. The slides were finally counterstained with Mayer's hematoxylin and washed once each with distilled water and PBS. Negative controls were stained with IgG1 and thyroid tissues were used as positive control [33].
Microscopic evaluation of thyroglobulin that was localized in both follicular epithelium and in the colloid was done according to its staining intensity [34].
Morphometric study
The thyroid glands from each rat in all groups were examined by objective lens with magnification ×400 under light microscopy using a Leica Qwin 500 image analyzer (Leica Ltd., Hessen, Germany). Faculty of Medicine, Zagazig University, Egypt. The measurement data were taken in 10 randomly selected nonoverlapping fields from each animal.
Using H&E stained sections
The area of thyroid follicles was measured to detect sizes of follicles, using the interactive measure menu.
Using Masson’s trichrome and PAS-stained sections
The mean collagen fibers area in the stroma between the thyroid follicles and the mean colloid percentage area were estimated. The section of the thyroid was enclosed inside the standard measuring frame in each randomly chosen field.
Using TGB immune stained sections
The immunereactive optical density for TGB intensity was determined.
Statistical Analysis
Results were expressed as mean ± standard deviation (SD). Multigroup comparisons of the means were carried out by one way analysis of variance (ANOVA) test. Least significant difference (LSD) test was used to compare the difference between the experimental groups and the control groups. The statistical significance difference for all tests was set at P<0.05 using SPSS software (v.16; Chicago, USA).
Results
Hormonal assay
The results of the present study revealed that, Propolis received group showed a non-significant difference in hormonal assay mean values when compared to control I and control II groups values (P>0.05) while the rats of CPF received group showed significant reduction in serum T3, T4 and TSH levels as compared to control I, control II and Propolis groups (Table 1). On the other hand, in CPF+Propolis group, the serum levels of T3,T4 and TSH showed significant increase as compared to CPF group (P<0.0001) as shown in Table 1. These results indicated CPF produced functional damage in both of excretory portion of thyroid gland represented by T3 and T4 and Pituitary hormonal pathway represented by TSH.
Oxidative and antioxidants assay
Compared to control I and control II groups, there was a nonsignificant difference in both of oxidative and antioxidants activities of Propolis treated group (P>0.05). On the other hand, there was significant increase in the contents of MDA levels and significant decrease in reduced GSH, GPX and SOD activities in thyroid tissues of CPF treated rats as compared to control I, control II and Propolis groups (Table 2). However, relative to CPF treated group, MDA content was significantly decreased and there was significant increase in reduced GSH,GPX and SOD activities in thyroid tissues of CPF+Propolis received group (P<0.0001) as shown in Table 2.
Histopathological evaluation of thyroid gland
As comparing the histopathological and the immunnohistochemical results in the control I and control II groups, the results showed similar findings. So, we used control I group to be compared with other groups (Figures 1-3).
| Groups | Control I | Control II | Group III (Propolis) | Group IV (CPF) | Group V (CPF+Propolis) |
|---|---|---|---|---|---|
| Parameter | mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD |
| T3 (ng/ml) | 51.2 ± 0.14 | 51.8±0.16 | 51.4 ± 0.18 | *44.28 ± 0.87 | **50.9 ± 0.17 |
| T4 (ng/ml) | 54.31 ± 0.14 | 54.60 ±0.22 | 54.25± 0.16 | *46.71 ± 0.19 | **54.53± 0.28 |
| TSH (pg/ml) | 0.57 ± 0.02 | 0.58 ± 0.02 | 0.56 ± 0.02 | *0.387 ± 0.01 | **0.522 ± 0.02 |
T3: Tri-iodothyron in, T4: Tetra-iodothyronin, TSH: Thyroid stimulating hormone
Results are expressed as mean ± SD of n=0 animals/group
P value of <0.05=Significant
*Significantly different compared to control (LSD) (P<0.0001)
**Significantly different compared to CPF group (LSD) (P<0.0001).
Table 1: Serum T3, T4 and TSH levels of different studied groups.
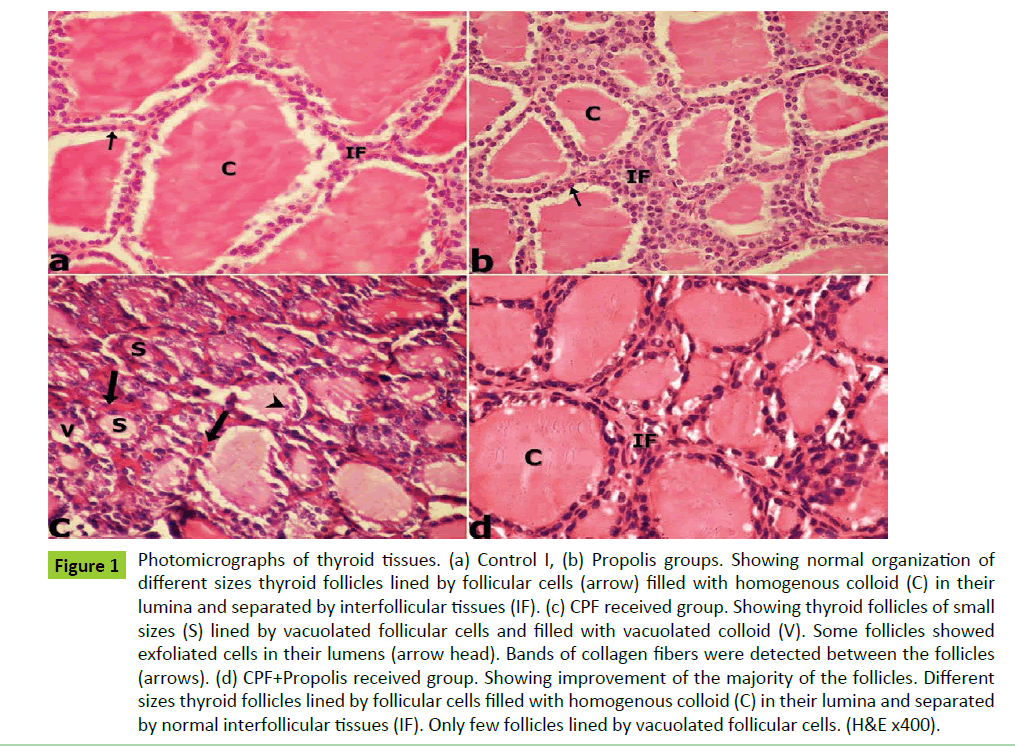
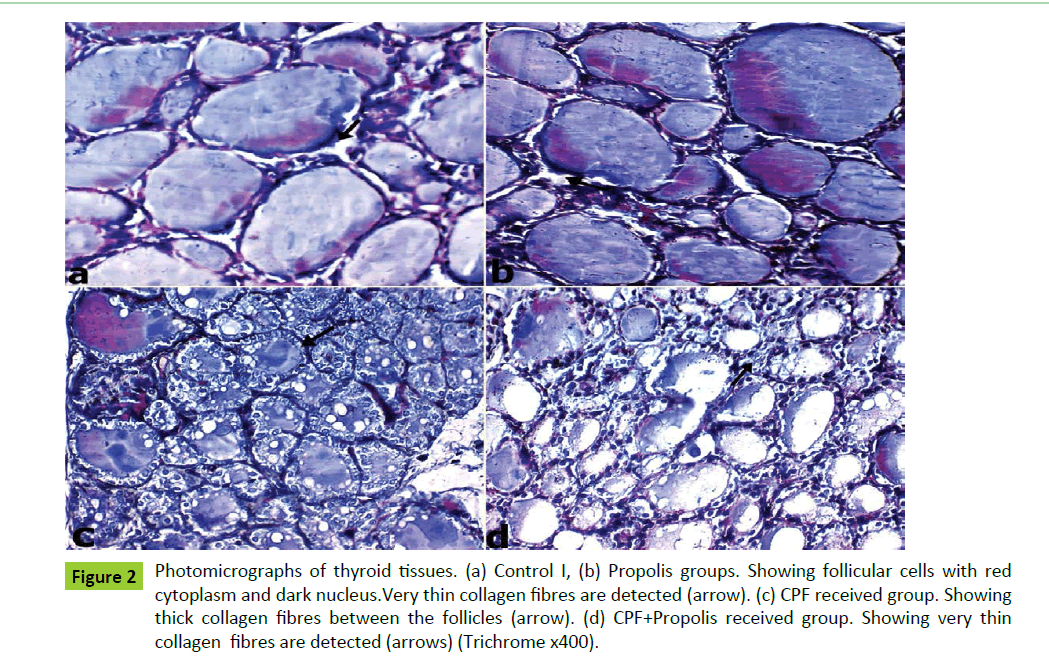
The histological examination of the thyroid glands of both control I and Propolis groups showed normal organization of variable size thyroid follicles that were filled with acidophilic homogenous colloid in their lumina and lined mainly by cubical follicular cells with round vesicular nuclei (Figures 1a and 1b). Interfollicular cells were detected between the follicles which separated by thin collagen fibers (Figures 2a and 2b).The colloid showed strong PAS reaction while the basement membrane showed moderate reaction (Figures 3a and 3b).
Examination of thyroid glands of CPF received group showed reduction in the size of most thyroid follicles, vacuolated follicular cells, vacuolated colloid and exfoliation of the follicular epithelial cells were also detected. (Figure 1c). The interfollicular tissues showed thickened collagen fibers (Figure 2c). The colloid showed moderate PAS reaction while basement membrane showed weak reaction (Figure 3c).
On the other hand on examination of thyroid gland sections of CPF+Propolis group showed improvement of the majority of the follicles in the form of disappearance of the colloid vacuoles and absence of exfoliation from follicles lumina. But still few follicles showed slight vaculation in the follicular cytoplasm (Figure 1d). The interfollicular tissues showed normal collagen as compared to control group (Figure 2d). Also, the colloid showed strong PAS reaction and basement membrane showed moderate reaction (Figure 3d).
The results of Immunnohistochemical evaluation of thyroid gland
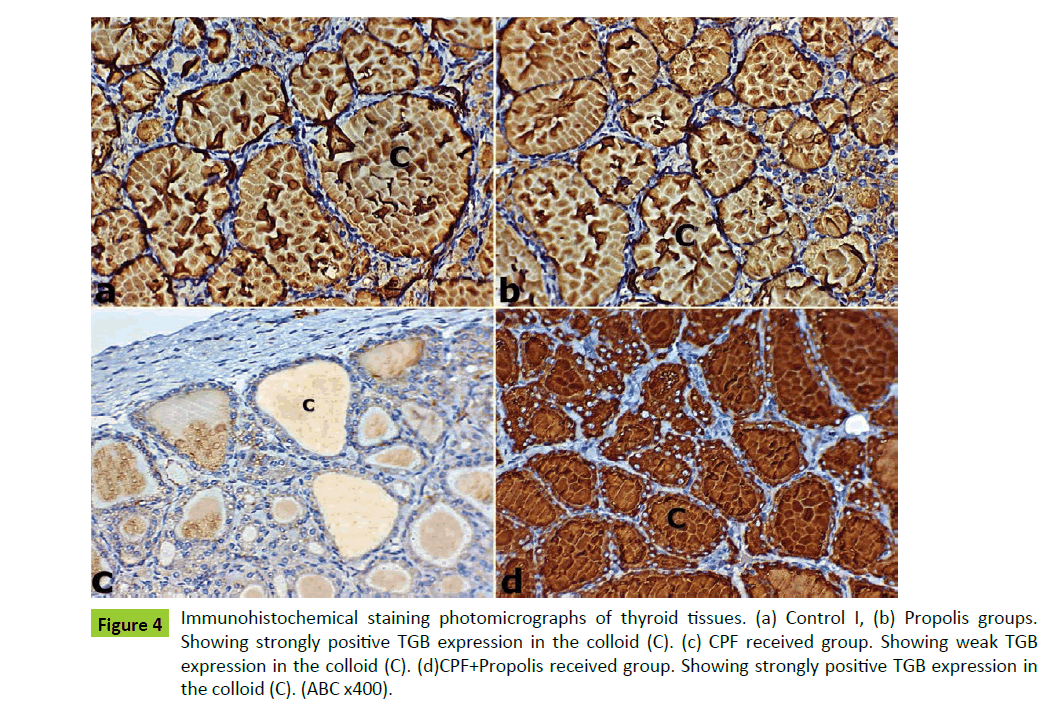
The thyroid sections of both control I and Propolis groups showed strong positive TGB staining intensity (Figure 4). These results appeared as marked brownish TGB expression in the colloid of thyroid follicles of both groups (Figures 4a). On examination of CPF received group sections group showed weak TGB expression in the colloid of thyroid follicles as compared to both control I and Propolis sections (Figure 4b).
On the other hand, the thyroid sections of CPF+Propolis received group showed marked brownish TGB expression in the colloid of thyroid follicles (Figures 4c and 4d).
Morphometric results
Hematoxylin and Eosin, Masson’s trichrome stain and PAS reaction were examined in thyroid tissues of studied groups (Table 3). Masson’s trichrome detection of fibrosis and PAS reaction for mucopolysaccharides contents of colloid results were shown in Table 3.
Propolis received group showed a non-significant difference in each of size of the thyroid follicles, collagen fibers percentage area and colloid percentage area (P>0.05) as compared to control I group. While there was highly significant increase of collagen fibers percentage area and reduction of both of colloid percentage area and thyroid follicles sizes in CPF received group sections as compared to control I group sections (P<0.0001). On other hand, CPF+Propoils received group sections showed highly significant reduction of collagen fibers percentage area and increased colloid percentage area and most of thyroid follicles sizes as compared to CPF received group sections (P<0.0001) (Table 4).
The mean optical density of TGB staining intensity for the studied groups is presented in Table 4. There was no significant difference between control I and propolis received groups in the optical density of TGB (P>0.05). On the contrary, there was highly significant difference in CPF received group as compared to control I group and highly significant difference in CPF+Propoils received group as compared to CPF received group (P<0.0001).
Discussion
Chlorpyrifos is the most common organophosphorus insecticide used in Egypt. Furthermore, the role of Propolis against CPF-induced thyroid toxicity has not been well studied. The present study aimed to evaluate CPF effects on thyroid gland and the possible ameliorating role of Propolis treatment in adult male albino rats. The present results showed that CPF administration for 12 weeks induced affection of thyroid gland structure and function as compared to control groups, where the histopathological results showed reduction in the size of some follicles and its amount of colloid, vacuolated colloid, exfoliation and vaculation of follicular cells. The interfollicular tissue showed thickened collagen and decreased PAS reaction. Also, immunohistochemical results showed weak thyroglobulin protein expression. These findings were confirmed with the hormonal assay results of CPF treated group that showed very highly significant reduction in thyroid hormones T3, T4 and TSH as compared to control groups.
These results are consistent with the findings of hypothyroidism in chlorpyrifos treated rats was also found by other researchers who reported decrease in the serum level of T3, T4 and significant increase in TSH in rats exposed to CPF [7, 35-37].
| Group | Control I | Control II | Group III (Propolis) |
Group IV (CPF) |
Group V (CPF+Propolis) |
|---|---|---|---|---|---|
| Parameter | mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD |
| MDA (nmol/g/tissue) | 1.55 ± 0.06 | 1.60 ± 0.06 | 1.59 ± 0.04 | *2.2 ± 0.27 | **1.62 ± 0.11 |
| GSH (mmol/g/tissue) | 16 ± 0.05 | 15.8 ± 0.44 | 15.9 ± 0.024 | *6.94 ± 0.20 | **15.8 ± 0.21 |
| GPX (U/g/protein) | 56.8 ± 0.08 | 56.5 ± 0.2 | 56.9 ± 0.4 | *40.7± 0.3 | **56.5 ± 0.2 |
| SOD (U/g/protein) | 69.3 ± 0.22 | 68.8 ± 0.30 | 69.1 ± 0.15 | *51.1 ± 0.93 | **68.7 ± 0.27 |
MDA: Malondialdehyde, GSH: Reduced Glutathione, GPX: Glutathione Peroxidase, SOD: Superoxide Dismutase Results are expressed as mean ± SD of n=10 animals/group
P value of <0.05=Significant
*Significantly different compared to control (LSD) (P<0.0001)
**Significantly different compared to CPF group (LSD) (P<0.0001).
Table 2: Tissue MDA, GSH, GPX and SOD levels in thyroid gland of different studied groups.
Figure 1: Photomicrographs of thyroid tissues. (a) Control I, (b) Propolis groups. Showing normal organization of different sizes thyroid follicles lined by follicular cells (arrow) filled with homogenous colloid (C) in their lumina and separated by interfollicular tissues (IF). (c) CPF received group. Showing thyroid follicles of small sizes (S) lined by vacuolated follicular cells and filled with vacuolated colloid (V). Some follicles showed exfoliated cells in their lumens (arrow head). Bands of collagen fibers were detected between the follicles (arrows). (d) CPF+Propolis received group. Showing improvement of the majority of the follicles. Different sizes thyroid follicles lined by follicular cells filled with homogenous colloid (C) in their lumina and separated by normal interfollicular tissues (IF). Only few follicles lined by vacuolated follicular cells. (H&E x400).
Figure 2:Photomicrographs of thyroid tissues. (a) Control I, (b) Propolis groups. Showing follicular cells with red cytoplasm and dark nucleus.Very thin collagen fibres are detected (arrow). (c) CPF received group. Showing thick collagen fibres between the follicles (arrow). (d) CPF+Propolis received group. Showing very thin collagen fibres are detected (arrows) (Trichrome x400).
Figure 3:Photomicrographs of thyroid tissues. (a) Control I, (b) Propolis groups. Showing strong PAS reaction in the colloid (C) and moderate reaction in basement membrane (arrow). (c) CPF received group. Showing moderate PAS reaction in both of the colloid (C) and the basement membrane (arrows). (d) CPP+Propolis received group. Showing strong PAS reaction in the colloid (C) and moderate reaction in basement membrane (arrow). (PAS x400).
Figure 4:Immunohistochemical staining photomicrographs of thyroid tissues. (a) Control I, (b) Propolis groups. Showing strongly positive TGB expression in the colloid (C). (c) CPF received group. Showing weak TGB expression in the colloid (C). (d)CPF+Propolis received group. Showing strongly positive TGB expression in the colloid (C). (ABC x400).
| Group | Control I | Group III (Propolis) |
Group IV (CPF) |
Group V (CPF+Propolis) |
|---|---|---|---|---|
| Parameter | mean ± SD | mean ± SD | mean ± SD | mean ± SD |
| Size of the follicles | 3317.7 ± 20.5 | 3146.5 ± 35.6 | 1523.9 ± 32.6 | 2996.9 ± 33.6 |
| Collagen fibers % area | 2.10 0.83 | 1.84 ± 0.71 | *6.57± 0.93 | **1.56 ± 0.44 |
| Colloid % area | 26.23 ± 2.41 | 25.88 ± 1.61 | *9.30 ± 1.07 | **24.88 ± 4.13 |
Results are expressed as mean ± SD of n=10 animals/group
P value of <0.05=Significant
*Significantly different compared to control (LSD) (P<0.0001)
**Significantly different compared to CPF group (LSD) (P<0.0001).
Table 3: Collagen fibers % area and Colloid % area in thyroid tissues of different studied groups.
| Group | Control I | Group III (Propolis) |
Group IV (CPF) |
Group V (CPF+Propolis) |
|---|---|---|---|---|
| Parameter | mean ± SD | mean ± SD | mean ± SD | mean ± SD |
| TGB intensity | 0.33 ± 0.04 | 0.36 ± 0.04 | *0.07 ± 0.02 | **0.31 ± 0.05 |
Results are expressed as mean ± SD of n=10 animals/group
P value of <0.05=Significant
*Significantly different compared to control (LSD) (P<0.0001)
**Significantly different compared to CPF group (LSD) (P<0.0001).
Table 4: Thyroglobulin optical density in thyroid tissues of different studied groups.
Chlorpyrifos damaging effects may be attributed to lipid peroxidation that could accelerate collagen synthesis by stimulating stellate cells which might cause thickened collagen fibres between thyroid follicles [38]. Weak thyroglobulin expression was explained by who reported that TGB is the thyrocytes synthesized glycoprotein and precursor for thyroid hormone [39]. TGB stored and secreted from the apical surface of the thyroid follicles, constituting the major component of colloid. TGB expression is diffuse in 100% of normal thyroid follicular epithelial cells.
The mechanisms contributing to CPF induced affection of thyroid hormones are not clear. Previous reports recorded that iodine binding proteins may be decreased by insecticides [40]. Also, several studies have shown that exposure to pesticides induced oxidative stress [41].
Also, reduction of thyroid hormonal assay may be attributed to the excessive production of reactive oxygen spices and free radicals in the central nervous system and its related glands, including the hypothalamic–pituitary axis, that may be one of the most important factors in the ageing of these structures and general aging process [42]. The aging of hypothalamic–pituitary axis leads to progressive functional loss and gradually develops into endocrine deficiency [43].
Furthermore, oxidative stress that is produced by formation of MDA and 4-hydroxynonenal adducts, leads to loss of elongation factor 2, which is an essential factor for protein synthesis in the hypothalamus and pituitary gland, leading to reduction of peptide hormones formation from the hypothalamic–pituitary axis [44].
In the line with potential role of CPF inducing thyroid toxicity via induction of oxidative stress, the results of the present study showed very highly significant increase in the contents of MDA and very highly significant decrease in the GSH, GPX and SOD activities in thyroid tissues of CPF treated rats. These results are in agreement with who reported enhancement of MDA production, decrease in reduced glutathione content, glutathione-S-transferase and catalase activities in rat tissues that treated with CPF [45]. Also, other previous studies have been reported accumulation of lipid peroxides in rat liver after exposure to acute dose of cholrpyrifos and kidney while, repeated doses of CPF increased LPO levels as well as decreased antioxidant enzymes in rat liver and lung [46-49].
Moreover, in the present work, the concurrent administration of Propolis with CPF attenuated the oxidative stress represented in significant reduction of MDA levels and improved the antioxidant defence represented in significant increase in the GSH, GPX and SOD activities in thyroid tissues compared to CPF group and to the extent approximated to control groups. These results confirmed its antioxidant protective role. This protective property was confirmed by the histopathological results of thyroid gland. Propolis nearly restored the normal sizes of the follicles, showed normalization of collagen and increased PAS reaction. It also, increased TGB immunostaining in the majority of thyroid sections. The serum levels of T3, T4 and TSH also showed very highly significant increase as compared to CPF administrated group.
These results are in agreement with who studied the effect of Propolis on CPF induced hepatic toxicity and reported serum reduction in thiobarbituric acid-reactive substances (TBARS) level and increased the activities of (SOD, CAT and GSH) [19]. Also with who studied the effect of Propolis on antioxidant status and reported that propolis caused reduction in the malondialdehyde (MDA) level and increased the activities of the antioxidant enzymes (SOD, GPX and CAT) [50,51].
Exposure to reactive oxygen species (ROS) leading to production of oxidative stress that outstrips the ability of the cell to remove them [50]. Tissue lipid peroxidation is a major consequence of the free radical-mediated injury and propagation because of high concentration of polyunsaturated fatty acids in cells [52].
The enzymatic antioxidant defence mechanism as superoxide dismutases, catalase and glutathione peroxidase minimize the cellular damage resulting from the interaction between ROS and cellular components [53].
Glutathione achieve its antioxidant activity through the thiol group that reduces disulfide bonds formed within cytoplasmic proteins to cysteines and provides antioxidant protection in the aqueous phase of cellular systems [54]. Also GSH is an important cofactor of deiodinases, the enzymes responsible for the conversion of thyroxine (T4) to triiodothyronine (T3) [55].
Therefore, oxidative stress that induced by CPF was related to thyroid hormonal derangement.
SOD catalyzes the dismutation process or partitioning of superoxide anion radical in to hydrogen peroxide and molecular oxygen. While GPX reduce lipid peroxides to the corresponding alcohols free hydrogen peroxide to water [56].
Antioxidants supplementation effectively suppressed the oxidative damage induced by OpI [57]. Propolis exerts its action through the scavenging of hydroxyl, superoxide free radicals and lipid peroxides [50]. The polyphenolic/flavonoid components of Propolis having anability to chelate metal ions and scavenge singlet oxygen, superoxide anions, peroxyl radicals, hydroxyl radicals and peroxynitrite [58].
Previous results were reported about the highest levels of flavonoides that librated from Propolis, occurred at highest concentration of ethanol during extract preparation [15].
Therefore, propolis flavonoids could increase the activities of the antioxidant enzymes and decrease the levels of the ROS.
Conclusion
In summary, from the foregoing results, the concurrent administration of Propolis with CPF improved the thyroid structural state and the hormonal level by restoration of the follicular sizes, normalization of collagen, increased PAS reaction, increased TGB immunostaining in the majority of thyroid sections and increased T3, T4 and TSH serum levels. It also, increased the antioxidants activities and reduced the ROS by increasing GSH, GPX and SOD activities in thyroid tissues and reduced MDA content. On the other hand, Chlorpyrifos exposure induced oxidative stress that produced thyroid toxic effects with subsequent reduction of thyroid hormones which important for most of physiological processes in adult male albino rats. It is recommended to use Propolis supplementation as antioxidant for overcoming the toxic effects of CPF and other OPI.
Acknowledgement
Great thanks and gratefulness to Prof. Dr. Mie Mohamed Samir Gomaa, Professor of forensic medicine and Toxicology, Zagazig University for her great support dedicating her effort, time and scientific experience in this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- John S, Kale M, Rathore N, Bhatnagar D (2001) Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem 12: 500-504.
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED (2008) Developmental diazinon neurotoxicity in rats: Later effects on emotional response. Brain Res Bull 75:166-172.
- Ambali SF, Joseph OA (2012) Vitamin C Attenuates chronic chlorpyrifos-induced alteration of neurobehavioral parameters in wistar rats. Toxicol Int 19:144–152.
- Slotkin TA1, Oliver CA, Seidler FJ (2005) Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Brain Res Dev Brain Res 157: 172-180.
- Yurumez Y, Cemek M, Yavuz Y Birdane YO, Buyukokuroglu ME (2007) Beneficial effect of N-acetylcysteine against organophosphate toxicity in mice. Biol Pharm Bull 30: 490–494.
- Andersen HR, Schmidt IM, Grandjean P et al (2008) Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environ Health Perspect 116: 566-572.
- De Angelis S, Tassinari R, Maranghi F (2009) Developmental exposure to chlorpyrifos induces alterations in thyroid and thyroid hormone levels without other toxicity signs in CD-1 mice. Toxicol Sci 108: 311-319.
- Meeker JD, Barr DB, Hauser R (2006) Thyroid hormones in relation to urinary metabolites of non-persistent insecticides in men of reproductive age. Reprod Toxicol 22: 437-442.
- Fliers E, Bianco AC, Langouche L, Boelen A (2015) Thyroid function in critically ill patients. Lancet Diabetes Endocrinol 3: 816-825.
- Ghisalberti EL (1979) Propolis: A review. Bee World 60:59-84.
- Valente MJ, Baltazar AF, Henrque R, Estevinho L, Carvalho M (2011) Biological activities of Portuguese propolis: Protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food Chem Toxicol 49: 86-92.
- Nirala SK, Bhadauria M (2008) Propolis reverses acetaminophen induced acute hepatorenal alterations: A biochemical and histopathological approach. Arch Pharm Res 31: 451-461.
- Yousef MI, Esmail AM, Baghdadi HH (2004) Effect of isoflavones on reproductive performance, testosterone levels, lipid peroxidation and seminal plasma biochemistry of male rabbits. J Environ Sci Health B 39: 819-833.
- Moreno MI, Isla MI, Sampietro AR, Vattuone MA (2000) Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol 71: 109-114.
- Park YK, Ikegaki M (1998) Preparation of water and ethanolic extracts of propolis and evaluation of the preparations. Biosci Biotechnol Biochem 62: 2230-2232.
- ILAR (Institute of Laboratory Animal Resources) (1996) Laboratory animal management: Rodents. Institute Of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, National Academy Press, Washington, DC.
- Bhadauria M, Nirala SK, Shukla S (2008) Multiple treatment of propolis extract ameliorates carbon tetrachloride induced liver injury in rats. Food Chem Toxicol 46: 2703-2712.
- Yousef MI1, Salama AF (2009) Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem Toxicol 47: 1168-1175.
- Newairy AA, Abdou HM (2013) Effect of propolis consumption on hepatotoxicity and brain damage in male rats exposed to chlorpyrifos. Afri J Biotechno 12: 5232-5243.
- Mansour SA, Mossa AM (2009) Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pesticide Biochem Physiol 93: 34-39.
- Hendawy AA, Zahra MH, Abd El-Aziz EA, Diab AA, Hamza RZ (2012) Ameliorative role and antioxidant effect of propolis and ginseng against reproductive toxicity of chlorpyrifos and profenofos in male rats. Life Sci J 9: 2557-2567.
- Goel A, Dani V, Dhawan DK (2005) Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem Biol Interact 156:131–140.
- Ambali SF, Dayom OA, Mufta S, Abdul Ganiyu G, Olushola OO, Joseph OA (2010) Chlorpyrifos-Induced clinical, hematological and biochemical changes in Swiss Albino mice- Mitigating effect by co-administration of vitamins C and E. Life Sci J 7: 37- 44.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882-888.
- Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158-169.
- Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46: 849-854.
- Clayden B (1971) Practical section cutting and staining. Fifth ed. Churchill Livingstone, Edinburgh and London, p: 270.
- Masson P (1929) Trichrome staining and their preliminary technique. J Tech Meth 12:75-90.
- Prophet EB, Mills B, Arrington JB, Sobin LH (1992) Laboratory methods in histotechnology. Fourth ed. Washington, DC 132: 53–58.
- McMANUS JF (1946) Histological demonstration of mucin after periodic acid. Nature 158: 202.
- Bancroft DJ, Gamble M (2008) Theory and Practice of Histological Techniques. Sixth Edn. New York 12: 121–123.
- Ramos-Vara JA, Beissenherz ME (2000) Optimization of immunohistochemical methods using two different antigen retrieval methods on formalin-fixed, paraffin-embedded tissues: Experience with 63 markers. J Vet Diagn Invest 12: 307–311.
- Wang YF, Biao L, Xiang-shan, F, Qiu R, et al (2015) Thyroid carcinoma showing thymus-like elements: A clinicopathologic, immunohistochemical, ultrastructural and molecular analysis. Amer J Clin Path 143: 223-233.
- Lamfon HA (2014) Effect of selenium on chlorpyrifos-induced thyroid toxicity in albino rats.
- Shady AM, Noor El-Deen FI (2010) Effect of chlorpyrifos on thyroid gland of adult male albino rats. Egy J Histo 33: 441–450.
- Ambali SF, Orieji C, Abubakar WO, Shittu M, Kawu MU (2011) Ameliorative effect of vitamin C on alterations in thyroid hormones concentrations induced by subchronic co administration of chlorpyrifos and lead in wistar rats. J Thyroid Res 2011:1-6. 214924.
- Hong RT, Xu JM, Mei Q (2009) Melatonin ameliorates experimental hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol 15:1452-1458.
- Liu H1, Lin F (2015) Application of immunohistochemistry in thyroid pathology. Arch Pathol Lab Med 139: 67-82.
- Goldner WS, Sandler DP, Yu F, Hoppin JA, Kamel F, et al. (2010) Pesticide use and thyroid disease among women in the Agricultural Health Study. Am J Epidemiol 171: 455-464.
- Jin YZ, hang X, Shu L, Chen L, Sun L, Qian H, Liu W, Fu Z (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphe 78: 846-852.
- McCann SM1, Mastronardi C, de Laurentiis A, Rettori V (2005) The nitric oxide theory of aging revisited. Ann N Y Acad Sci 1057: 64-84.
- Hertoghe T (2005) The “multiple hormone deficiency” theory of ageing: Is human senescence caused mainly by multiple hormone deficiencies? Ann NY Acad Sci 1057: 448–465.
- Vitale G1, Salvioli S, Franceschi C (2013) Oxidative stress and the ageing endocrine system. Nat Rev Endocrinol 9: 228-240.
- Ahmed MM, Zaki NI (2009) Assessment the ameliorative effect of pomegranate and rutin on chlorpyrifosethyl-induced oxidative stress in rats. Nature and Science 7: 49-61.
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ (1995) In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicol 104: 129-140.
- Oncu M, Gultekin F, Karaöz E, Altuntas I, Delibas N (2002) Nephrotoxicity in rats induced by chlorpryfos-ethyl and ameliorating effects of antioxidants. Hum Exp Toxicol 21: 223-230.
- Bebe FN, Panemangalore M (2003) Exposure to low doses of endosulfan and chlorpyrifos modifies endogenous antioxidants in tissues of rats. J Environ Sci Health B 38: 349-363.
- Akhgari M, Abdollahi M, Kebryaeezadeh A, Hosseini R, Sabzevari O (2003) Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for toxicity of malathion in blood and liver of rats. Human Exp ToxicoL 22: 205-211.
- Jasprica I, Mornar A, Debeljak Z, Smolcić-Bubalo A, Medić-Sarić M, et al. (2007) In vivo study of propolis supplementation effects on antioxidative status and red blood cells. J Ethnopharmacol 110: 548-554.
- Cerutti PA (1985) Prooxidant states and tumor promotion. Science 227: 375-381.
- Verma, RS, Mehta A, Srivastava N (2007) In vivo chlorpyrifos induced oxidative stress: Attenuation by antioxidant vitamins. Pesticide Biochem Physiol 88: 191-196.
- Kanbur M, Eraslan G, Silici S (2009) Antioxidant effect of propolis against exposure to propetamphos in rats. Ecotoxicol Environ Saf 72: 909-915.
- El-sharaky AS, Newairy AA, Kamel MA, Eweda SM (2009) Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rat. Food Chem Toxicol 47: 1584-1590.
- Chopra IJ (1997) Clinical review 86: Euthyroid sick syndrome: Is it a misnomer? J Clin Endocrinol Metab 82: 329-334.
- Bhabak KP, Mugesh G (2010) Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc Chem Res 43: 1408–1419.
- Elhalwagy MEA, Darwish NS, Zaher EM (2008) Prophylactic effect of green tea polyphenol against liver and kidney injury induced by fenitrothion insecticides. Pesti Bio Physio 91: 81-89.
- Fonseca YM, Marquele-Oliveira F, Vicentini FT, Furtado NA, Sousa JP, et al. (2011) Evaluation of the potential of Brazilian propolis against UV-induced oxidative stress. Evid Based Complement Alternat Med.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences