Protective Effect of 3, 3-Diindolylmethane on Bisphenol A Activated GPR30, RAS, PI3K/Akt Estrogenic Signaling Pathway in Female Sprague-Dawley Rats
Subbaiyan Thilagavathi, Pachaiappan Pugalendhi, Thangarasu Rajakumar and KrishnamoorthyVasudevan
DOI10.21767/2471-9641.100026
Subbaiyan Thilagavathi1, Pachaiappan Pugalendhi1*, Thangarasu Rajakumar1and KrishnamoorthyVasudevan2
1Department of Biochemistry and Biotechnology, Annamalai University, India
2Department of Zoology (DDE), Annamalai University, India
- *Corresponding Author:
- Pugalendhi P
Department of Biochemistry and Biotechnology
Faculty of Science, Annamalai University Annamalainagar
Tamil Nadu, India.
Tel: +914144239141
E-mail: pugalau@gmail.com
Received date: May 23, 2017; Accepted date: June 19, 2017; Published date: June 23, 2017
Citation: Thilagavathi S, Pugalendhi P, Rajakumar T, Vasudevan K (2017) Protective Effect of 3, 3-Diindolylmethane on Bisphenol A Activated GPR30, RAS, PI3K/Akt Estrogenic Signaling Pathway in Female Sprague- Dawley Rats. J Med Toxicol Clin Forensic Med. Vol. 3 No. 1: 6
Abstract
The present study was aimed to evaluate the protective effect of 3, 3-diindolylmethane (DIM) on bisphenol A (BPA) induced estrogen signaling in the mammary glands of female Sprague-Dawley rats. Chronic administration of BPA (10 μg/kg/bw) to rats exerts rapid estrogenic action via GPR30 and activate cascade of signaling molecule which induce cell proliferation in the mammary gland. Western blot analysis of mammary tissues shows over expression of GPR30, SRC, RAS, PI3K and Akt proteins and immunohistochemical analysis reveals over expression of PCNA and no significant changes in ERs. Further, oral administration of DIM (5 mg/kg/bw), alternative days to BPA treated rats for the period of 12 weeks show significant decrease in the expression pattern of GPR30, SRC, RAS, PI3K, Akt and PCNA. The results of our study demonstrate that BPA induces rapid action via the over expression of proteins in non-genomic estrogen signaling pathway. Administration of DIM inhibits the action of BPA by modulating the expression of proteins involved in GPR30 cascade signaling pathway mediated cell proliferation in mammary glands of female rats.
Keywords
GPR30; Mammary gland; DIM; Bisphenol A; Sprague-Dawley rats
Introduction
Currently public and scientific attentions have been focused on environmental chemicals having endocrine disruptive activity. Endocrine disrupting chemicals are ubiquitous in the environment which alters normal function and signaling effect of hormones. Bisphenol A (BPA) is well-known industrial chemical, acts as a xenoestrogen because of its structural resemblance with 17-β estradiol, an active form of estrogen. BPA disrupts the normal action of 17-β estradiol by interfering with estrogen signaling pathway [1].
Endogenous estrogen signaling mainly occurs in liganddependent/ genomic pathways. Estrogen ligand directly binds to estrogen receptors (ER-α/ER-β) which translocates in to nucleus where ER-DNA interaction regulates the expression of estrogen responsive genes or through a tethering mechanism in which ERs tether to DNA with other transcription factors. The binding affinity of ER and transcription apparatus is facilitated by coactivators. The nuclear receptor co-activator family (p160 family) of protein such as steroid receptor co activators such as steroid receptor co-activator 1, 2, 3 (SRC1, SRC2, SRC3) act as binding protein for the assembly of complex machinery required for chromatin remodeling and RNA synthesis [2].
Ligand-independent/non-genomic pathway involves the activation of other signal transduction pathways that lead to rapid responses, within minutes of estrogen exposure. The mechanism of non-genomic ER signaling is thought to be mediated by membrane-associated G-protein-coupled receptor known as GPR30 which mediates rapid estrogen signaling independent of ERs [3]. Further, GPR30 induces mitogen activated protein kinase (MAPK) or phosphatidylinositol kinase (PI3K) signaling pathway changes in intracellular calcium or stimulation of cAMP production [4-7]. Diethylstilbestrol (DES) also induces rapid estrogen signaling via non-genomic ER signaling pathway. Most of the environmental chemicals are bind to GPR30 and express the similar effect of ERs [8]. A number of studies reveal that the BPA exert rapid estrogen signaling via binding to GPR30 [9,10].
Di-indolyl methane (DIM) is a plant indole found in cruciferous vegetables such as broccoli, Brussels sprouts, cabbage, cauliflower and kale. It has potential estrogen-modulating and antineoplastic activities. DIM is a stable derivative of indole-3-carbinol (I3C), which promotes estrogen metabolism by increasing the formation of 2-α hydroxy estrogen metabolites, thereby increasing antioxidant, antiestrogenic and antiandrogenic activities [11- 13]. DIM shows anticarcinogenic effects by controlling cell cycle, cell proliferation in hormone dependent as well as independent cancer [14] (Figure 1).
The previous study reported that Hippo signaling and Akt pathway controlling cell proliferation by PI3K inhibitor and DIM treatment in colon cancer cells [15]. In addition, DIM induced G1 cell cycle arrest that coincided with a strong induction of P21 gene expression and promoter activity in both estrogen responsive and nonresponsive breast cancer cells [16]. In this study, we explore the protective effect of DIM against the low dose of BPA activated GPR30 mediated alteration in PI3K-Akt pathway in Sprague-Dawley rats.
Material and Methods
Chemicals
Bisphenol A (BPA), diindolylmethane (DIM), were purchased from Sigma Aldrich chemicals Pvt. Ltd., Bangalore, India. Antibodies for GPR30, SRC, RAS, PI3K, AKT, ER, PCNA and β-actin were procured from Santa Cruz Biotechnology, USA. All the other chemicals used were of analytical grade.
Animal model
Six to seven weeks old female Sprague-Dawley rats (weighed 100-120 g), were purchased from National Institute of Nutrition, Hyderabad, India. Rats were maintained in the Central Animal House, Raja Muthiah Medical College and Hospital, Annamalai University, Chidambaram, Tamil Nadu, India. The experimental protocol was approved by the institutional animal ethics committee by the Committee for the Control and Supervision of Experimental Animal (CPCSEA Approval no: 1028). The rats were maintained under controlled condition of temperature (24 ± 2ÃÆââ¬Â¹Ãâà ¡C) humidity (50 ± 10%), 12 h light/dark cycle feed and water provided ad libitum.
Experimental design
Total numbers of 24 rats were randomly divided into four groups and each group contains six rats. Group I: Control (vehicle treated) rats received 1 ml of corn oil for 12 weeks, group II: Rats received BPA alone (10 μg/kg bw dissolved in 1 ml of corn oil for 12 weeks), group III: Rats received DIM control (1.0 ml corn oil+5 mg/kg, bw/alternative days, in 1 ml of 2% DMSO, orally), group IV: Rats received BPA (10 μg/kg bw in 1 ml of corn oil)+DIM (5 mg/kg, bw/alternative days, in 1 ml of 2% DMSO, orally).
At the end of 12th week, rats were fasted overnight and sacrificed by cervical decapitation. The mammary tissues were immediately subdivided and processed for distribution to each experiment.
Immunohistochemistry
Paraffin embedded mammary tissue sections were processed and then immunostained with the primary antibodies for ER-α, and PCNA (proliferating cell nuclear antigen) at a concentration of 1 μg/ml with 3% BSA in TBS and incubated overnight at 4ÃÆââ¬Â¹Ãâà ¡C. The slides were washed thrice with TBST, and incubated with their respective HRP conjugated secondary antibodies, diluted 1:200 with 3% BSA in TBS and incubated for 2 h at room temperature. Sections were then washed with TBST and incubated with 0.02% diaminobenzidine containing 0.01% hydrogen peroxide for 5-10 min and counter staining was performed using haematoxylin and the slides were visualized under a light microscope. The percentage of the positively stained cells were semi-quantitatively analysed by using image J, software.
Western blotting analysis
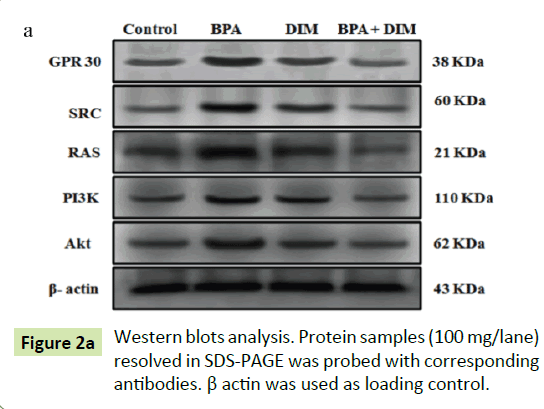
Mammary tissues were homogenized in ice-cold RIPA buffer. The homogenate was centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant was collected and the protein concentration was measured by the method of Lowry et al. [17]. Total cellular proteins containing 50 μg were loaded and separated using 10% SDS polyacrylamide gel electrophoresis. The separated proteins were transferred into PVDF membrane. The membranes were incubated in blocking buffer containing 5% BSA (Bovine Serum Albumin) for 2 h to reduce non-specific binding sites and then incubated with the specific primary antibodies for GPR30, SRC, Ras, PI3K, Akt, and β-actin were diluted with 5% BSA in TBS (Tris- Buffered Saline) for overnight at 4°C. Membranes were washed thrice with TBST (Tris-Buffered Saline Tween 20) for 10 min each. The membranes were incubated with their corresponding secondary antibodies for 2 h at room temperature. Protein bands were visualized by an enhancing chemiluminescence method using an ECL kit (GenScript ECL kit, USA). Bands were scanned using a scanner and quantitated by Image J, a public domain Java image processing software, Wayne Rasband, NIH, USA, which control was set to 1.
Statistical analysis
Statistical analysis was performed using SPSS 16 (SPSS, Inc., Chicago) statistical package. The data are expressed as mean ± standard deviation (SD). One way analysis of variance (ANOVA) followed by Duncan Multiple Range Test (DMRT) comparison method was used to correlate the difference between the variables. Data are considered statistically significant if p values are less than 0.05.
Results
Effect of DIM on BPA induced protein expression of GPR30, SRC, RAS, PI3K and AKT in mammary tissues of control and experimental rats
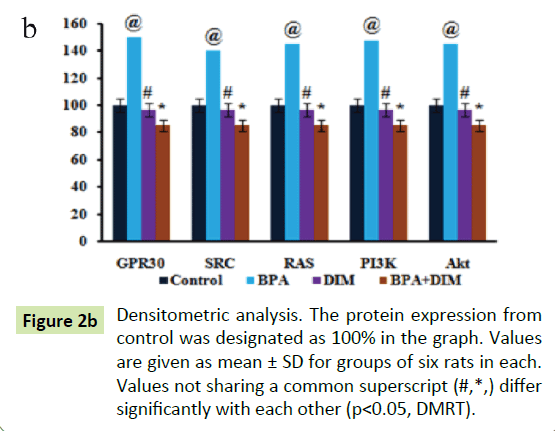
Figure shows the western blot analysis of GPR30, SRC, Ras, PI3K and Akt protein expression in mammary tissues of control and experimental rats. The histogram analysis reveals the expression of GPR30, SRC, RAS, PI3K and Akt are significantly (p<0.05) increased in group II BPA alone treated rats when compared to group I control rats. Whereas the expression of GPR30, SRC, RAS, PI3K and Akt are significantly (p<0.05) decreased in group IV BPA and DIM treated rats when compared to the group II BPA alone treated rats. However, there are no significant (p<0.05) changes in the expression pattern of GPR30, SRC, RAS, PI3K and Akt in group III DIM alone treated animals when compared to group I control animals (Figure 2).
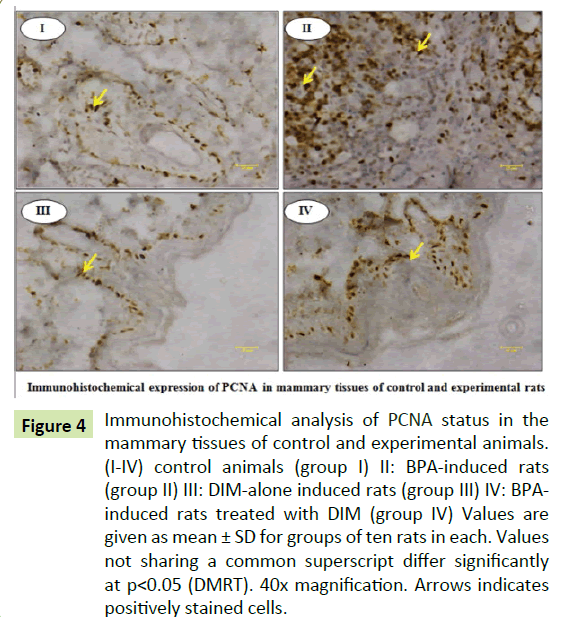
Effect of DIM on BPA induced expression of ER-α and PCNA in mammary tissues of control and experimental rats
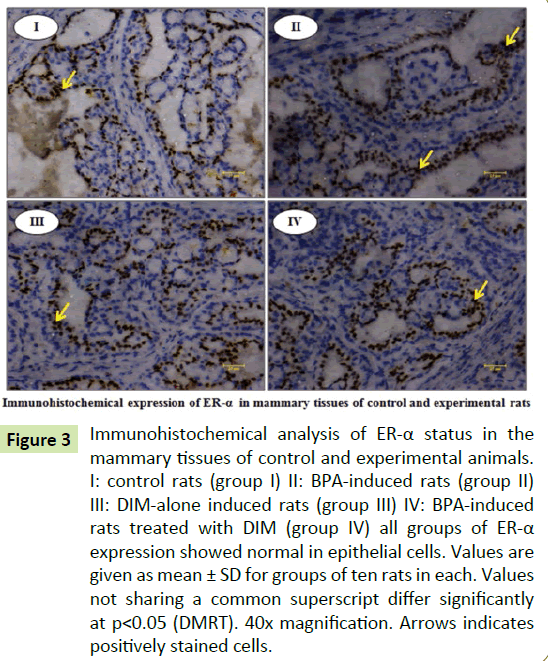
Figures show the immunohistochemical expressions of ER-α and PCNA in the mammary tissues of control and experimental rats. The expression of PCNA are significantly (p<0.05) increased in group II BPA treated rats when compared to group I control rats. Whereas the expression of PCNA are significantly (p<0.05) decreased in group IV BPA and DIM treated rats when compared to group II BPA alone treated rats. However, there are no significant (p<0.05) changes in the expression of PCNA in group III DIM alone treated rats when compared to group I control rats. Further, we observed no significant changes in the expression of ER-α in all groups (Figures 3 and 4).
Figure 3:Immunohistochemical analysis of ER-α status in the mammary tissues of control and experimental animals. I: control rats (group I) II: BPA-induced rats (group II) III: DIM-alone induced rats (group III) IV: BPA-induced rats treated with DIM (group IV) all groups of ER-α expression showed normal in epithelial cells. Values are given as mean ± SD for groups of ten rats in each. Values not sharing a common superscript differ significantly at p<0.05 (DMRT). 40x magnification. Arrows indicates positively stained cells.
Figure 4:Immunohistochemical analysis of PCNA status in the mammary tissues of control and experimental animals. (I-IV) control animals (group I) II: BPA-induced rats (group II) III: DIM-alone induced rats (group III) IV: BPAinduced rats treated with DIM (group IV) Values are given as mean ± SD for groups of ten rats in each. Values not sharing a common superscript differ significantly at p<0.05 (DMRT). 40x magnification. Arrows indicates positively stained cells.
Discussion
Phytochemicals and its derivative DIM have been reported to modulate many gene expression involved in controlling cell cycle, cell proliferation, signal transduction and apoptosis [18,19]. The relationship between 2-α hydroxyl estrone and 16-α hydroxy estrone metabolite is crucial to determine its pathological effects. Increasing the ratio of 2-α hydroxyl estrone to 16-α hydroxyl estrone, may reduce the risk of estrogen-related cancer. DIM favorably alter estrogen metabolism and helps to increase the ratio of 2:16 estrogen metabolites [20].
GPR30 is a G-protein coupled transmembrane receptor responsible for non-genomic signaling of estrogen and estrogenlike chemicals. Recently GPR30 is considered as an estrogen receptor hence it is called as G-protein estrogen receptor (GPER) [21]. But its mode of functions is obviously differing from those of the classical nuclear ERs. Several studies were reported that the GPR30 mediates the effect of several xenoestrogens through rapid activation of signal transduction pathways [22]. BPA and other endocrine disruptors have high binding affinity to GPR30. BPA act as a mitogenic agent inducing mammary gland cell proliferation, promoting growth and puberty in rats [23,24]. The recent study reported that BPA-induced cell proliferation occurs via JAK/Stat3, MAPK/ERK and PI3K/Akt pathways [25]. BPA can exert Erk1/2/c-fos signaling through GPR30 in SKBR3 cells and up-regulate the expression of an AP1-target gene pS2 via a pathway induced by Erk1/2/c-fos signaling [26]. In this study, western blot analysis showing over expression of GPR30, SRC, Ras, PI3K and Akt and immunohistochemical analysis shows increased expression of PCNA in mammary glands of BPA alone treated rats. These results are finds in line with above studies.
The activation of p160 SRC family proteins (SRC1, SRC2 and SRC3) occurs by interacts with nuclear receptors and specific transcription factors [27]. They initiate chromatin remodeling and activation of enzymes which facilitate assembly of transcriptional apparatus for transcriptional activation, SRC activation is vital for mitogenic responses of growth factors, such as epidermal growth factor or platelet-derived growth factor [28]. The increased expression of SRC has been implicated in breast cancer susceptibility [29]. The multitude of signals such as estrogen, growth factor are activate are SRC-3 which plays a vital role in breast cancer cell proliferation, invasion and metastasis through the over expression of ER-α, EGFR and MMPs. Recent study also reported that increased SRC activity has been associated with carcinogenesis and inhibition of SRC family kinases can decrease the renal interstitial fibroblasts proliferation [30]. In transgenic mice, over expression of SRC-3 shows an increased susceptibility to mammary cancer [31]. SRC-3/A1B1-deficient mice also show reduced Akt activity and over activation of Akt occur in SRC-3/ A1B1-over expression mice [32]. Over expression of SRC1-3 observed in prepubertal BPA exposure without altering ER-α in rat mammary tissues [33]. In this study also over expression of SRC family proteins are observed.
Under normal condition, the cell proliferation is maintained through GPR30 includes the mitogen-activated protein kinase (MAPK) mainly extracellular signal-regulated kinase (ERK) and PI3K pathways. Ligands binding to their respective receptor activate SRC which in turn leads to activation of matrix metalloproteinases (MMPs) which release the active form of heparin-binding epidermal growth factor (HB-EGF) [34,35]. This transactivates the epidermal growth factor receptor (EGFR), leading to the activation of Ras which ultimately activates Akt kinase through PI3K kinase [36]. Akt regulates the expression of estrogen responsive genes controlling cell proliferation, growth survival, and metastasis [37]. Atrazine, an environmental chemical having estrogenic potential induce the expression of estrogen specific target genes and cell proliferation via GPR30/EGFR/MAPK signal transduction pathway. Atrazine not directly bind and activate ER-α, along with GPR30 is important for estrogen-like activity [38]. Immunohistochemical analysis of PCNA, a cell proliferative marker, was used to examine the proliferative response of BPA in mammary epithelial cells in rats. BPA exposure stimulates mammary gland epithelial cell proliferation leads to hyperplasia in BRCA1 mutant mice [39].
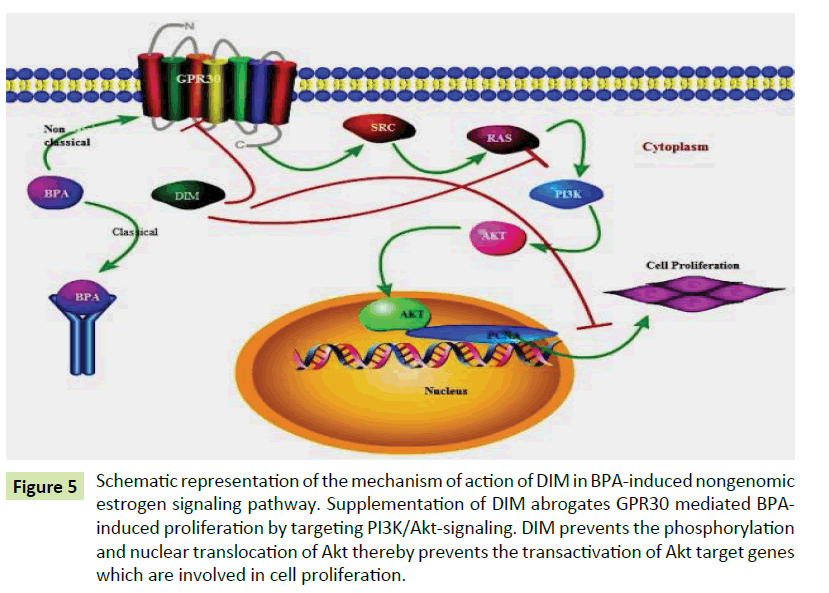
DIM inhibit angiogenesis by modulating VEGF receptor signaling through down-regulating ERK1/2 activity. RAS-GTP content was decreased with DIM treatment, resulted in the reduced activities of Raf and MAPK kinase [40]. In this study, oral administration of DIM to BPA treated rats normalized the expression of Ras, PI3K, Akt protein level and PCNA expression. Phytochemicals reduce the adverse effects of E2 and its metabolites. Chen et al. reported that DIM given as 5 mg/kg, every other day inhibited DMBA induced rat mammary tumor growth and volume that was not accompanied by induction of hepatic CYP1A1-dependent activity [41]. DIM or its precursor I3C, have been found to inhibit estrogeninduced genes [12]. DIM decreasing the E2 induced LNCaP cells proliferation [42]. Rajoria et al. demonstrate that DIM inhibits cell proliferation by combined suppression of ERK and Akt pathway [43]. Particularly, DIM targets and inhibits the phosphorylation of key molecules ERK 1/2 and Akt and also targets the in vitro metastasis of HT-29 (50-TS) cells as evidenced by decreased migration, adhesion and invasion of these cells. Garikapatty et al. reported that DIM induces the down-regulation of Akt, p-Akt and PI3 kinase [44]. Wang et al. reported that DIM inhibits the growth of breast cancer cell lines that are over-expressing Her-2 and activated Akt via the modulation of the PI3K/Akt pathway and p27Kip1 independent of estrogen receptor status [45]. Hence, DIM has the potential to inhibit cell proliferation in the mammary gland of low dose BPA treated rats via modulating the activation of GPR30 mediated PI3K cascade signaling molecules involved in cell proliferation. The putative mechanism of action of DIM on the GPR30 is given in the diagram (Figure 5).
Figure 5:Schematic representation of the mechanism of action of DIM in BPA-induced nongenomic estrogen signaling pathway. Supplementation of DIM abrogates GPR30 mediated BPAinduced proliferation by targeting PI3K/Akt-signaling. DIM prevents the phosphorylation and nuclear translocation of Akt thereby prevents the transactivation of Akt target genes which are involved in cell proliferation.
Conclusion
To conclude, the BPA (10 μg/kg bw) induces activation of GPR30 and activate SRC which in turn activate MMPs and EGFR which further leads to over expression of RAS. RAS promote the up regulation of PI3K and Akt. Being a plant indole, DIM can induce anti estrogenic responses through the ligand-independent activation of ERs prevent the up-regulated expression of a phosphorylated form of PI3K-Akt activities and hence protect the abnormal state of cell proliferation. Our study revealed that treatment of DIM protected the BPA induced abnormal mammary cell proliferation via PI3K-Akt signaling pathway. This study may be beneficial to prevent the occurrence of estrogen induced breast cancer.
Conflicts of Interest
All authors declare they have no conflicts of interest.
Acknowledgement
We gratefully acknowledge the financial assistance from University Grants Commission (UGC), New Delhi, India in the form of Major Research Project.
References
- Shanle EK, Xu W (2011) Endocrine disrupting chemicals targeting estrogen receptor signaling identification and mechanisms of action. Chem Res Toxicol 24: 6-19.
- Bulynko, YA, O'Malley BW (2011) Nuclear receptor co-activators: Structural and functional biochemistry. Biochemistry 50: 313-28.
- Lombardi M, Castoria G, Migliaccio A, Barone MV, Di Stasio R, et al. (2008) Hormone dependent nuclear export of estradiol receptor and DNA synthesis in breast cancer cells. J. Cell Biol 182: 327-40.
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM (2011). Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut 159: 212-8.
- Faivre EJ, Lange CA (2007) Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol 27: 466-80.
- Ajj H, Chesnel A, Pinel S, Plenat F, Flament S, et al. (2013) An alkylphenol mix promotes seminoma derived cell proliferation through an ER alpha36-mediated mechanism 8: e61758.
- Björnström L, Sjöberg M (2005) Mechanisms of estrogen receptor signaling convergence of genomic and non-genomic actions on target genes. Mol Endocrinol 19: 833-842.
- Thomas P, Dong J (2006). Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol 102: 175-179.
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, et al. (2008) Pancreatic insulin content regulation by the estrogen receptor ER alpha 3: e2069.
- Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC (2005) Signaling from the membrane via membrane estrogen receptor-alpha: Estrogens, xenoestrogens and phytoestrogens. Steroids 70: 364-371.
- Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF (2004) Pilot study effect of 3,3-di-indolyl methane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer 50: 161-167.
- Chen I, Hsieh T, Thomas T, Safe S (2001) Identification of estrogen-induced genes downregulated by AhR agonists in MCF-7 breast cancer cells using suppression subtractive hybridization. Gene 262: 207-214.
- Chang X, Tou JC, Hong C, Kim HA, Riby JE, et al. (2005) 3, 3-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 26: 771-778.
- Nachshon-Kedmi M, Fares FA, Yannai S (2004) Therapeutic activity of 3,3-di-indolyl methane on prostate cancer in an in vivo model. Prostate 61: 153-160.
- Li XJ, Leem SH, Park MH, Kim SM (2013) Regulation of YAP through an Akt-dependent process by 3,3'-di-indolyl methane in human colon cancer cells. Int J Oncol 43: 1992-1998.
- Chibo Hong, Hyeon A, Kim Gary L, Firestone, Leonard F (2002). 3,3'-Diindolylmethane (DIM) induces a G1 cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of P21 WAFI/CIP1 expresion. Carcinogenesis 23: 1297-1305.
- Lowry OH, Rosebrough MJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-Phenol reagent. J Biol Chem 193: 265-275.
- Sarkar FH, Li Y (2004) Indole-3-carbinol and prostate cancer. J. Nutrit 134: 3493S-3498S.
- Li Y, Li X, Sarkar FH (2003) Gene expression profiles of I3Cand DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. J Nutr 133: 1011–1019.
- Taioli E, Im A, Xu X, Veenstra TD, Ahrendt G, et al. (2010). Comparison of estrogens and estrogen metabolites in human breast tissue and urine. Reprod Biol Endocrinol 2: 8-93.
- Prossnitz ER, Barton M (2011) The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7: 715-726.
- Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, et al. (2006) Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res 12: 6359-6366.
- Maffini MV, Rubin BS, Sonnenschein C, Soto AM (2006) Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol Cell Endocrinol 254-255: 179-186.
- Keri RA, Ho SM, Hunt PA, Knudsen KE, Soto AM, et al. (2007) An evaluation of evidence for the carcinogenic activity of bisphenol-A. Reprod Toxicol 24: 240–252.
- Anna Ptak, Marta Hoffmann, Izabella Gruca, Justyna Barc (2014) Bisphenol-A induce ovarian cancer cell migration via the MAPK and PI3K/Akt signaling pathways. Toxicol Lett 229: 357–365.
- Dong S, Terasaka S, Kiyama R (2011) Bisphenol-A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut 159: 212-218.
- DeMali KA, Kazlauskas A (1998) Activation of Src family members is not required for the platelet-derived growth factor beta receptor to initiate mitogenesis. Mol Cell Biol 18: 2014-2022.
- Roche S, Fumagalli S, Courtneidge SA (1995) Requirement for Src family protein tyrosine kinases in G2 for fibroblast cell division. Science 269: 1567-1569.
- Jun Wang, Sarah Jenkins, Lamartiniere CA (2014) Cell proliferation and apoptosis in rat mammary glands following combinational exposure to bisphenol-A and genistein. BMC Cancer.
- Yan Y, Ma L, Zhou X, Ponnusamy M, Tang J, et al. (2015) Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int 89: 68-81.
- Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, et al. (2004) High tumor incidence and activation of the PI3K / AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6: 263-274.
- Kuang SQ, Liao L, Wang S, Medina D, O’Malley BW, et al. (2005) Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res 65: 7993-8002.
- Jenkins S, Wang J, Eltoum I, Desmond R, Lamartiniere CA et al. (2011) Chronic oral exposure to bisphenol A results in a nonmonotonic dose response in mammary carcinogenesis and metastasis in MMTV-erbB2 mice. Environ Health Perspect 119: 1604-1609.
- Li YR, Ren CE, Zhang Q, Li JC, Chian RC (2013). Expression of G protein estrogen receptor (GPER) on membrane of mouse oocytes during maturation. J Assist Reprod Genet 30: 227-232.
- Mo Z, Liu M, Yang F, Luo H, Li Z, et al. (2013) GPR30 as an inhibitor of tamoxifen resistance in hormone-dependent breast cancer. Breast Cancer Res 15: 114.
- Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, et al. (2013) G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet Gynecol Int.
- Peyton C, Thomas P (2011) Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated trough the G protein-coupled estrogen receptor (GPER) in zebrafisch (Danio rerio). Biol Rep 85: 42-50.
- Albanito L, Lappano R, Madeo A, Chimento A, Prossnitz ER, et al. (2008) G protein-coupled receptor 30 and estrogen receptor alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ Health Perspect 116: 1648-1655.
- Laundette P, Jones, Aishia Sampson, Hyo Jin Kang, et al. (2010) Loss of BRCA1 leads to an increased sensitivity to Bisphenol A. Toxicol Lett 199: 261-268.
- Chang X, Firestone GL, Bjeldanes LF (2006) Inhibition of growth factor-induced Ras signaling in vascular endothelial cells and angiogenesis by 3,3-di-indolyl methane. Carcinogenesis 27: 541-550.
- Chen I, McDougal A, Wang F, Safe S (1998) Aryl hydrocarbon receptor-mediated anti-estrogenic and anti-tumorigenic activity of diindolylmethane. Carcinogenesis 19: 1631-1639.
- Smith S, Sepkovic D, Bradlow HL, Auborn KJ (2008) 3, 3'-Diindolylmethane and genistein decrease the adverse effects of estrogen in LNCaP and PC-3 prostate cancer cells. J Nutri Biochem 138: 2379-2385.
- Rajoria S, Suriano R, Wilson YL, Schantz SP, Moscatello A, et al. (2011) 3,3'-di-indoly lmethane inhibits migration and invasion of human cancer cells through combined suppression of ERK and AKT pathways. Oncol Rep 25: 491-497.
- Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. (2006) 3,3'-Di-indoly lmethane downregulates pro-survival pathway in hormone independent prostate cancer. Biochem Biophys Res Commun 340: 718-725
- Wang Z, Yu BW, Rahman KM, Ahmad F, Sarkar FH (2008) Induction of growth arrest and apoptosis in human breast cancer cells by 3,3-di-indolyl methane is associated with induction and nuclear localization of p27kip Mol Cancer Ther 7: 341-349.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences