Histopathological and Biochemical Effects of Acute and Chronic Tramadol Drug Toxicity on Liver, Kidney and Testicular Function in Adult Male Albino Rats
Heba Youssef S and Azza HM Zidan
DOI10.21767/2471-9641.10007
1Forensic Medicine and Clinical Toxicology, Faculty of Medicine Port Said University, Port Said, Egypt
2Pathology departments, Faculty of Medicine Port Said University, Port Said, Egypt
- Corresponding Author:
- Heba Youssef S
Forensic Medicine and Clinical Toxicology
Faculty of Medicine Port Said University
Port Said, Egypt
Tel: 202-0663210400
Fax: 202-0663670320
E-mail: vivek03sharma@rediffmail.com
Received date: October 07, 2015, Accepted date: October 21, 2015, Published date: October 28, 2015
Citation: Youssef SH, Zidan AHM. Histopathological and Biochemical Effects of Acute and Chronic Tramadol Drug Toxicity on Liver, Kidney and Testicular Function in Adult Male Albino Rats. J Med toxicol clin forensic med. 2016, 1:2.
Abstract
Background: Nowadays tramadol is becoming abused more popular among teens in most countries worldwide; especially between males. The aim of present study was to investigate the histopathological and biochemical profiles of acute and chronic toxic effects of tramadol hydrochloride on hepatic, renal and testicular functions.
Materials and methods: Sixty male adult albino Sprague-Dawley rats were used in this experimental study. Rats were divided into three equal groups. Each group contained twenty rats. Group I: served as control group. Group II: representing acute tramadol toxicity and group III: representing tramadol dependent use daily for 60 days.
Results: Histopathological results regarding hepatic tissues of group II displayed hemorrhage and cytolysis in the hepatocytes. In group III hepatic tissue showed complete cell membrane degeneration of hepatocytes when both groups compared to group I. Renal tissues in group II showed glomerular hemorrhage while in group III there was atrophied glomeruli with collapsed tufts, wide Bowman's space, degenerated tubules and cellular infiltration when both groups compared to group I. The histopathological examination of testicular tissues revealed atrophy of seminiferous tubules with interstitial calcification in group II. The histopathological lesions were inform of focal testicular degeneration with single or multiple layer of vacuolated spermatocytes, with a little evidence of spermatogenesis in group III when both groups were compared to group I. Biochemical results indicated that the levels of liver enzymes specific (ALT, AST and ALP) and serum bilirubin were significantly increased in group II and III when compared to the control group. Similarly for renal function, the levels of creatinine and blood urea nitrogen (BUN) were also significantly increased in group II and III when compared to control group. The sex hormones levels were significantly increased (estradiol (E2) and prolactin (PRL) compared to control group , while tramadol administration caused a significant decrease in testosterone level with a gradual reduction in luteinizing hormone ( LH) and follicular stimulating hormone ( FSH) as compared to control group.
Conclusion: Evidence of histopathological and biochemical affection of hepatic, renal and sexual function evoked by acute toxicity of tramadol and its repeated administration for long periods. Recommendations: The necessity of designing a national awareness campaign to the public especially the youth to spotlights on the health hazards of tramadol abuse.
Keywords
Tramadol, Histopathological changes, Biochemical indices, Hepatotoxicity, Nephrotoxicity, Sexual Dysfunction, Rats
Introduction
Nowadays addiction is an increasing social and health problem worldwide despite all efforts to prevent and control it. Analgesics are among the most popular drugs which are being abused [1].
Tramadol is a centrally acting opioid analgesic which is mainly used for the treatment of moderate to severe pain [2]. Its efficiency and potency ranges between weak opioids and morphine [3]. Tramadol is rapidly absorbed orally; Tramadol has extensive tissue distribution. The bioavailability of tramadol is 70% its bioavailability is more than that of morphine (15- 65%) when tramadol is used in multiple or repeated doses, the bioavilability increased to 100%. The complete absorption of tramadol takes place in the upper part of the small intestine. The plasma concentrations of tramadol vary with its form to reach a peak concentration within two hours in capsule form and five for tablets. Tramadol metabolism occurs in liver by the cytochrome P450 enzyme system and its by-products are excreted unchanged through the kidneys. Repeated tramadol administration might lead to the accumulation of toxic metabolites in the body, increase the risk for its toxicokinetics effects and/ or decrease the clearance of tramadol, thus increasing its potential for toxicity [4].
Tramadol abuse, dependence as well as acute overdose -related deaths have been increasingly reported especially in young male adults [5]. Being an opioid, tramadol carries all the possible risks known from other opiates [6]. Tramadol can cause psychological and physical addiction similar to that seen with other opiates and opioids [7]. Recently young addicts typically substituted tramadol for heroin. Repeated tramadol administration in such patients might lead to accumulation of toxic metabolites in their bodies, increase the risk for pharmacokinetics interactions and or decrease the clearance of tramadol thus increasing its potential for toxicity [8].
So the aim of this work was to investigate, the impact of acute and chronic administration of tramadol hydrochloride on the histopathological and biochemical profiles in hepatic, renal and testicular functions in adult male albino rats.
Materials and Method
Drug: Tramal® (Tramadol HCl), 50 mg capsules, was obtained from Mina- Pharm, Egypt. Its chemical name is (+) cis-2- [(dimethylamino)methyl]-1-(3-m ethoxyph-enyl) cyclohexanol hydrochloride.
Animals, dosing and experimental design: Sixty male adult albino Sprague-Dawley rats, their body weight ranged from 180 to 200 gm purchased from center for experimental animals, Faculty of Veterinarian Medicine, Zagazig University were used in the study. This study was performed in accordance with the Guide for the ethical care and use of laboratory animals (1985), NIH, Bethesda. All rats were left to acclimatize for one week prior to the experiment and were housed in plastic cages maintained at controlled room temperature (22-24 C) with 12 hour diurnal (day and night change) with free access to standard pellet animal diet and tap water.
The animals were divided into three main groups. Each group contained twenty rats for each group as follows:
Group I: Control group which is further subdivided into subgroup IA and IB.
Subgroup IA: (n=10 rats) animals received 1ml/day normal saline (0.9% NaCl) orally by gavage. They were kept for 24 hours as the rats of group II.
Sub group IB: (n=10 rats) animals received 1ml/day normal saline (0.9% NaCl) orally by gavage. They were kept throughout the experiment under the same conditions for sixty days as the rats of group III.
Group II: (Tramadol acute toxicity group n=20 rats): animals received a single LD50 dose (300mg/kg body weight) of tramadol hydrochloride orally by gavagev [9].
Group III: (Tramadol dependent group n=20 rats): animals received tramadol hydrochloride in gradually increasing doses until it reached the dependent dose in sixty days. Dependence was induced by giving the therapeutic dose of tramadol hydrochloride which was calculated according to Paget’s equation. The therapeutic dose for rat weighting 200 gm=18/1000 x adult human therapeutic daily dose (400 mg) =7.2 mg [10]. Then the dose was gradually increased by adding the initial calculated therapeutic dose every three days till reaching (144 mg) at the end of sixty days.
The calculated tramadol hydrochloride doses were delivered in normal saline and given orally to each animal by a curved needle –like oral tube that was introduced directly into stomach (a gavage process). At the end of the experiment, surviving animals of the all groups were sacrificed by cervical dislocation at 24 hours after a single dose for group II (acute toxicity group) and their control group IA and after the last dose at the end of the sixty days for group III (dependent group) and their control group IB. Autopsy was carried out for all animals of the all groups. The hepatic, renal and testicular tissues were fixed in 10% formalin solution for histopathological examination.
I-Histopathological examination: The selected organs for each rat were received at the Pathology department, Faculty of Medicine; Port Said University preserved in 10% formalin solution and dehydrated in ascending grades of alcohol. After xylene treatment, the specimens were embedded in paraffin blocks. Five-micron thick; serial sections were cut by microtome and stained with hematoxylin and eosin (H&E). The stained sections were examined using light microscope according to Bancroft and Gamble [11]. Microscopic examinations were carried out in all selected organs of acute tramadol toxicity and dependant group. Unintentional bias was prevented by coding rats’ tissue samples. Liver sections were assessed for the followings; a) Paranchymal changes including steatosis, feathery degeneration, necrosis, congestion, hemorrhage, interface hepatitis, Kupffer cell hyperplasia and sinusoidal dilation, b) Portal changes including inflammatory cellular infiltrates, fibrosis and bile duct proliferation. Kidney sections were assessed for the following glomeruli, visceral and parietal layers of Bowman´s capsule. Testicular section was assessed for spermatogenic cells and the number of spermatozoa in seminiferous tubules.
II-Biochemical indices: Blood samples were collected in dry centrifuge tubes for serum prepration, sera were separated and preserved at -20°C till used for biochemical analysis to detect liver, kidney and sexual hormonal levels.
A-Liver function indices
Aminotransferases, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes activities were measured in IU/l using the method of Thomas [12]. Serum alkaline phosphatase (ALP) enzyme in IU/l and bilirubin level in mg/100ml were measured based on the method of Akiyuki Ohkubo [13] and Perry [14].
B-Kidney function indices
Creatinine (Cr.) level in mg/dl was determined using the method of Fossati [15]. Blood urea nitrogen (BUN) measurement in mg/dl was assessed based on the cleavage of urea with urease (Berthelot´s reaction) according to Orsonneau, Massoubre and Cabanes [16].
C-Sex hormones assay
Luteinizing hormone (LH) and follicle stimulating hormone (FSH) in mU/ml, prolactin (PRL) in mg/ml , testosterone in pg/ml and esradiol (E2) in μg/ml were determined using enzyme linked immunosorbant assay (ELISA) kits according to manufacture structure.
III-Statistical Analysis: All the data was expressed as mean ± standard deviation (SD) and analyzed using Statistical Package for Social Sciences (SPSS) program version 20. Kolmogorof seminerof method was applied for all groups to find out if the data was non parametric.Since all the data was parametric, all the comparisons among groups were carried out using one way Analysis of Variance (ANOVA) followed by Bonferroni post hoc test. Data were considered statistically significant with P ≤ 0.05.
Results
A) Histopathological results (Figure 1-3)
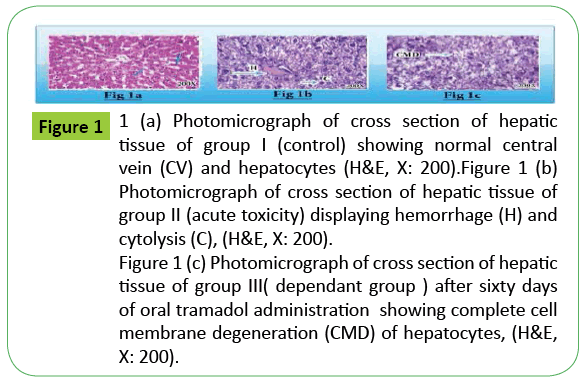
Figure 1: 1 (a) Photomicrograph of cross section of hepatic
tissue of group I (control) showing normal central
vein (CV) and hepatocytes (H&E, X: 200).Figure 1 (b)
Photomicrograph of cross section of hepatic tissue of
group II (acute toxicity) displaying hemorrhage (H) and
cytolysis (C), (H&E, X: 200).
Figure 1 (c) Photomicrograph of cross section of hepatic
tissue of group III( dependant group ) after sixty days
of oral tramadol administration showing complete cell
membrane degeneration (CMD) of hepatocytes, (H&E,
X: 200).
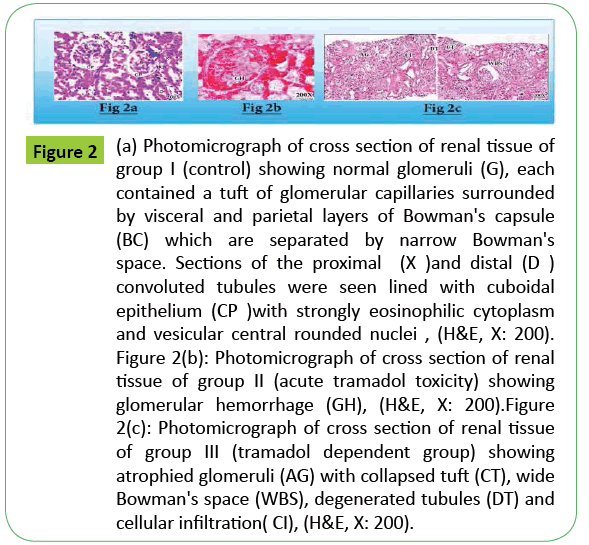
Figure 2: (a) Photomicrograph of cross section of renal tissue of group I (control) showing normal glomeruli (G), each contained a tuft of glomerular capillaries surrounded by visceral and parietal layers of Bowman's capsule (BC) which are separated by narrow Bowman's space. Sections of the proximal (X )and distal (D ) convoluted tubules were seen lined with cuboidal epithelium (CP )with strongly eosinophilic cytoplasm and vesicular central rounded nuclei , (H&E, X: 200). Figure 2(b): Photomicrograph of cross section of renal tissue of group II (acute tramadol toxicity) showing glomerular hemorrhage (GH), (H&E, X: 200).Figure 2(c): Photomicrograph of cross section of renal tissue of group III (tramadol dependent group) showing atrophied glomeruli (AG) with collapsed tuft (CT), wide Bowman's space (WBS), degenerated tubules (DT) and cellular infiltration( CI), (H&E, X: 200).
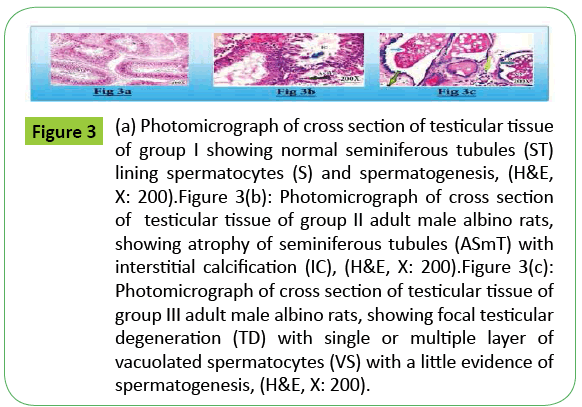
Figure 3: (a) Photomicrograph of cross section of testicular tissue of group I showing normal seminiferous tubules (ST) lining spermatocytes (S) and spermatogenesis, (H&E, X: 200).Figure 3(b): Photomicrograph of cross section of testicular tissue of group II adult male albino rats, showing atrophy of seminiferous tubules (ASmT) with interstitial calcification (IC), (H&E, X: 200).Figure 3(c): Photomicrograph of cross section of testicular tissue of group III adult male albino rats, showing focal testicular degeneration (TD) with single or multiple layer of vacuolated spermatocytes (VS) with a little evidence of spermatogenesis, (H&E, X: 200).
B) Biochemical indices results (Table 1-5)
| Studied Groups | Liver Enzymes level in IU/L | F | P | LSD | |
| AST IU/l Mean ±SD |
ALT IU/L Mean ±SD |
||||
| Group I (N=20) | 34.7± 2.8 | 123±2.3 | 9.4 | ||
| Group II (N=20) | 66.45+2.35 | 216.95+2.50 | 31.67 | 0.001** | II vs. I |
| Group III (N=20) | 79.80+1.25 | 228.75+1.90 | 45.1 | 0.000** | III vs. I |
SD the standard deviation. ** P<0.01 highly significant F=One way Analysis of Variance (Fissure test). LSD difference shows significant difference between N=number of rats in each group
Table 1 shows highly significant increase in the mean values of serum AST, ALT in group II and III when compared with group I.
Table 1: Effects of tramadol acute toxicity and daily dosing for 60 days on liver enzymes (AST & ALT) in adult albino rats.
| Studied Groups | ALP and Bilirubin levels | F | P | LSD | |
|---|---|---|---|---|---|
| ALP IU/l Mean ±SD |
Bilirubin (mg/100ml) Mean ±SD |
||||
| Group I (N=20) | 50.3± 0.30 | 0.21± 0.05 | 50.09 | ||
| Group II (N=20) | 83.1± 0.29 | 0.50± 0.06 | 82.6 | 0.001** | II vs. I |
| Group III (N=20) | 94.8± 80.2 | 0.65± 0.03 | 94.1 | 0.000** | III vs. I |
** P<0.01 highly significant F=One way Analysis of Variance (Fissure test). LSD difference shows significant difference between N=number of rats in each group.
Table 2 shows a highly significant increase in the mean alkaline phosphatase enzyme and bilirubin levels in group II and III when compared with group I.
Table 2: Effects of tramadol acute toxicity and daily dosing orally for 60 days on liver alkaline phosphatase enzymes (ALP) & bilirubin levels in adult albino rats .
| Studied Groups | serum creatinine level in mg/dl Mean ±SD |
F | P | LSD |
|---|---|---|---|---|
| Group I (N=20) | 0.42±0.19 | 0.6 | ||
| Group II (N=20) | 0.60±0.32 | 2.8 | 0.001** | II vs. I |
| Group III (N=20) | 0.63±0.35 | 2.9 | 0.001** | III vs. I |
** P<0.01 highly significant F=One way Analysis of Variance (Fissure test). LSD difference shows significant difference between N=Number of rats in each group.
Table 3: Analysis Of Variance (ANOVA) for the effects of tramadol acute toxicity and daily dosing for 60 days on the serum creatinine level in adult male albino rats.
| Studied Groups | Mean blood Urea level (mg/dl) Mean ±SD |
F | P | LSD |
|---|---|---|---|---|
| Group I(N=20) | 34.3±21.5 | |||
| Group II(N=20) | 44.7±7.7 | 9.6 | 0.001** | II vs. I |
| Group III(N=20) | 51.6±1.9 | 12.7 | 0.001** | IIIvs. I |
** P<0.01 Highly Significant F=One way Analysis of Variance (Fissure test). LSD difference shows significant difference between N=Number of rats in each group.
Table 4 shows highly significant increase in the mean values of blood urea level in group II and III when compared with group I.
Table 4: Analysis Of Variance (ANOVA) for the Effects of tramadol acute toxicity and daily dosing for 60 days on blood urea level in adult male albino rats.
| Studied Groups | Sex hormones Mean ±SD |
F | P | LSD | ||||
| LH mlU/ml |
FSH mlU/ml |
Testosterone (mg/ml) |
Estradiol (pg/ml) |
Prolactin (μg/ml) |
||||
| Group I N=20 |
2.40+0.20 | 2.20+0.15 | 4.40+0.44 | 46.30+1.55 | 6.90+1.35 | |||
| Group II N=20 |
1.35+0.35 | 0.85+0.30 | 1.80+0.60 | 53.95+0.19 | 15.95+1.12 | 9.6 | 0.001** | II vs. I |
| Group III N=20 |
0.76+0.15 | 0.55+0.11 | 0.85+0.13 | 62.0+0.25 | 19.00+1.55 | 12.7 | 0.001** | IIIvs. I |
** P<0.01 Highly Significant F=One way Analysis of Variance (Fissure test). LSD difference shows significant difference between N=Number of rats in each group.
Table 5 shows a highly significant decrease in LH, FSH, testosterone hormones in group II and III when compared with group I. There is highly significant increase in the mean value of estradiol and prolactin levels in group II and III when compared with group I.
Table 5: Analysis Of Variance (ANOVA) for the effects of tramadol acute toxicity and daily dosing orally for 60 days in the sex hormones levels on adult male albino rats.
Discussion
Analgesics are the most commonly consumed over- the-counter preparation all over the world. Tramadol hydrochloride, a synthetic analogue of codeine, is a centrally acting analgesic drug [1]. After its introduction in the 70s, tramadol gained great interest because it is prescribed for moderate to severe pain [17]. Nowadays tramadol is becoming abused more popular among teens in most countries worldwide and especially between males. So the present study was conducted to investigate, the histopathological and biochemical profiles of acute and chronic toxic effects of tramadol hydrochloride in liver, kidneys and testicular functions on adult male albino rats.
The present study revealed that histopathological examination of liver tissue of group II displayed necrosis and cytolysis , while liver tissue of group III showed complete cell membrane degeneration of hepatocytes when both groups compared with control group I.
These results could be explained by the fact that the liver is responsible for the metabolism and excretion of tramadol [18]. Tramadol hydrochloride is mainly metabolized in liver by N-and
Table 3 shows a highly significant increase in the mean values of serum creatinine level in group II and III when compared with group I.
O-demethylation, followed by conjugation with glucuronic acid and sulphate. The active metabolite, O-desmethyl tramadol shows higher affinity for mu-opioid receptors and has twice the analgesic potency of the parent drug [5].
Similar results were demonstrated by experimental studies of acute and long term treatment of morphine in mature albino rats which revealed that morphine can produce hepatotoxic effects during its metabolism in the form of necrosis; hemorrhage and cytolysis were also documented in morphine treated rats. However they noted only perivenular hydropic degeneration in the hepatic tissue [19].
Another study demonstrated that the postoperative effects of morphine and tramadol on the histopathology of liver in rabbits which had undergone isoflurane anesthesia, hepatocyte degeneration, central vein dilatation, and mononuclear cellular infiltration in the morphine and tramadol group were more severe than those of the control group. In addition, sinusoidal dilatation and cell membrane degeneration of the hepatocytes were more in tramadol group than that of the morphine group. These results suggest that morphine and tramadol may lead to some changes in liver tissue [20].
The liver histopathological changes in the current study proved the acute and chronic hepatotoxic effects of tramadol in adult albino rats. The hepatic histopathological results in the present study pointed out the risk of increased hepatic damage due to acute and chronic use of tramadol.
The liver histopathological effects of acute and chronic tramadol toxicity in the current study were supported by the liver function indices results. There were highly significant increase in serum AST, ALT, alkaline phosphatase enzyme and bilirubin levels in group II and III when compared with group I.
These results were comparable with the findings of [21] who reported increased ALT, AST activities in rats after acute and long term administration of morphine like agent Levo-alphaacetylmethadol HCL (LAAM) and also among heroin users and similar to the results of El-Gaafarawi [22] who recorded a significant increase in the ALT and AST activities in rats after administration of 40 mg/kg b.wt. and 80 mg/kg b.wt. tramadol than control ones. Alkaline Phosphatase ALP enzyme present in cell surface in most human tissues. The highest concentration is found in the intestine, liver, bone, spleen and kidney [23] and Moss and Handerson [24]. The specific location of the enzyme with both sinusoidal and bile canalicular membranes accounts for the more predominant elevations in certain disorders as observed in the present study with tramadol administration. Impaired secretion of hepatic ALP may be accompanied by acute cell necrosis, so liberation of ALP in the circulation is elevated. The cellular injury may still persist as indicated by increased AST, ALT, ALP and bilirubin activities.The findings of the current investigation were in agreement with those of Sebnem et al., [24] who reported that the levels of ALT, AST, ALP and bilirubin were significantly higher in rats exposed to acute and gradual increasing doses of morphine till reaching dependency when compared to the control group.
Studying of the histopathological effects of acute and chronic toxicity of tramadol on kidney tissue of group II revealed glomerular hemorrhage, while kidney tissue of group III showed atrophied glomeruli with collapsed tufts, wide Bowman's space, degenerated tubules and cellular infiltration when compared with control group I.
This could be explained by the toxicokinetics process of tramadol, since 30% of the drug is excreted through the kidney in an unchanged manner. While the rest are changed into active metabolites by the liver. Metabolites of the drug that are excreted via kidneys may also cause cellular damage leading to kidney dysfunction [25].
The kidney histopathological results of the present work was supported by the kidney function indices results which showed a highly significant increase in the mean values of serum creatinine and blood urea BUN levels in group II and III when compared with group I.
This increase in serum creatinine and BUN levels with acute and chronic tramadol dosing, is also in accordance with results of Atici [26]. In rats receiving morphine for a month. Similarly El-Gaafarawi [22] stated that morphine and codeine administration for a long period resulted in an increased creatinine and BUN levels. The above observations may be confirmed by the suggestions of Wu [27] who stated that liver and kidney are responsible for tramadol metabolism and excretion, so it may cause hepatotoxicity and nephrotoxicity.
Testicular tissue of group II in the current study showed atrophy of semeniferous tubules with interstitial calcification while testicular tissue of group III showed focal testicular degeneration with single or multiple layers of vacuolated spermatocytes with a little evidence of spermatogenesis.
The wide abuse of tramadol drug among young people especially male gender led to investigate the histopathological effects of acute and chronic use of tramadol in the testicular function in adult male albino rats. The present study showed atrophy of semeniferous tubules with interstitial calcification among group II and focal testicular degeneration with single or multiple layers of vacuolated spermatocytes with a little evidence of spermatogenesis among group III.
This is in agreement with Caju [28] who observed reduction of Sertoli and Leydig cells in mature albino rats exposed to acute and chronic doses of morphine. They explained this testicular change by disorders in the endocrine and paracrine functions that can indirectly influence the final size of sertoli cell population through disordered LH, estradiol, somatotropin, somatostatin, prolactin and GnRH (gonadotropin-releasing hormone) acted upon either on the hypothalamus or directly on pituitary glands. In addition to opiate induced reduction of serum testosterone level; there is abnormal structural and functional abnormalities of the secondary sex organs. It seems that probably opiates could affect testicular volume and induce arrested spermatogenesis, sloughed germinal epithelium, destroyed Sertoli cells and thickened and irregular basement membrane as well as signs of apoptosis [29].
Recent studies by Abou El Fatoh [30] revealed that the testes undergo severe diffused testicular degeneration with numerous spermatocytes and spermatid giant cell formation (the cells might be fused together to form such giant cells) without spermatogenesis after tramadol in a dose of 40 mg/kg b.wt. The spermatocytes were mostly necrotic.
The abnormalities observed in the testicular structures in the present study including atrophy of semeniferous tubules with interstitial calcification in group II and focal testicular degeneration with single or multiple layers of vacuolated spermatocytes with a little evidence of spermatogenesis in group III might be attributed to the oxidative damaging effect of free radicals since the testicular cells and sperms contain abundant polyunsaturated fatty acids in their plasma membranes. Lipid peroxidation induced by tramadol can eventually result in dysfunction and structural damage of cells [31].
The testicular histopathological results of group II and III in the current study was supported by sex hormonal dysfunction evident by a highly significant decrease in LH, FSH, testosterone hormones and a highly significant increase in the mean value of estradiol and prolactin levels when compared with group I.
Opiate use is known to decrease the levels of male sex hormones and this lowered hormonal level is thought to be responsible for the diminished fertility of male opiate users Yassa [32]. In the present study, gonadal examinations revealed that administration of acute toxic dose of tramadol, and daily doses for two months influenced reduced sex hormones activity of male rats compared to control group. Tramadol influenced this activity where there was a reduction in the levels of LH, FSH and testosterone with induction of PRL and E2 levels. Previous studies concerned with gonadal activity during drugs abuse have been supported the present results where Tennese and Wevrick [33] reported decreased levels of LH and testosterone with increased prolactin hormone after morphine and methadone administration. Also El- Gaafarawi [22] observed the reduction of serum levels of LH, FSH and testosterone and the induction of prolactin hormone (PRL) and E2 secretions after cannabis use. Similar results for reduced testosterone and elevated E2 have been reported by Abdellatief [34].
Conclusion
The current study proved that tramadol has acute and chronic toxic effects on the structure and function of hepatic, renal and testicular tissues of male albino Sprague-Dawley rats. Therefore it is suggested that tramadol as reported more effective in pain management, yet its toxic effects should be kept in mind.
Recommendations
The potential effects of long term tramadol therapy on the liver, kidney and testes should be explained to patients who are under treatment with tramadol for long time before the therapy is commenced.
Monitoring of liver and kidney functions tests as well as sex hormones are recommended. If there are abnormalities; tramadol hydrochloride should be tapered and withdrawn, if this is clinically acceptable. If opioid therapy has to continue, male sex hormone replacement therapy should be initiated and monitored by an endocrinologist.
References
- Rafati A, Yasini SM ,Norani F, Mohammad HDR, Saeed P (2012) World J. Med. Sci 1: 40-43.
- Nossaman VE, Ramadhyani U, Kadowitz PJ, Nossaman BD (2010) Advances in perioperative pain management: Use of medications with dual analgesic mechanisms, tramadol &tapentadol. AnesthesiolClin 28: 647-666.
- Miranda HF (2013) Antinociception tolerance and physical dependence comparison, between morphine and tramadol .Pharmacology Biochemistry and Behavior 71: 389-395.
- Shadnia S, Soltaninejad K, Heydari K, Sasanian G, Abdollahi M (2008) Tramadol intoxication: a review of 114 cases. Hum ExpToxicol 27: 201-205.
- Lee HJ, Cha KE, Hwang SG, Kim JK, Kim GJ (2011) In vitro screening system for hepatotoxicity: comparison of bone-marrow-derived mesenchymal stem cells and Placenta-derived stem cells. J Cell Biochem 112: 49-58.
- Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, et al. (2005) Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994-2004. Pharmacoepidemiol Drug Saf 14: 851-859.
- Senay EC, Adams EH, Geller A, Inciardi JA, Muñoz A, et al. (2003) Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend 69: 233-241.
- Tjaderborn M (2013) Fatal unintentional intoxication with tramadol .Forensic SciInt 175: 109-115.
- Matthiesen T, Wöhrmann T, Coogan TP, Uragg H (1998) The experimental toxicology of tramadol: an overview. ToxicolLett 95: 63-71.
- Paget GE, Barnes JM (1964) "Evaluation of Drug Activities and Pharmacometrics". Laurence, DR. and Bacharach, AL. (edition), Academic Press, London.
- Bancroft JD, Gamble M (2002) "Theory and Practice of Histological Technique". (5th edition), Churchill Livingstone, Edinburg, London.
- Thomas L (2007) Alanine aminotransferase (ALT), aspartate aminotrasferase (AST): in Thomas, L. editor. Clinical Laboratory Diagnostics (1st ed), TH-Books Verlagsgesellschaft, Frankfurt.
- Akiyuki Ohkubo (1974) Rat liver alkaline phosphates assay .Journal of biochemistry 249: 7174-7180.
- Perry BW, Doumas BT, Bayse DD, Butler T, Cohen A, et al. (1983) A candidate reference method for determination of bilirubin in serum. Test for transferability. ClinChem 29: 297-301.
- Fossati P, Prencipe L, Berti G (1983) Enzymiccreatinine assay: a new colorimetric method based on hydrogen peroxide measurement. ClinChem 29: 1494-1496.
- Orsonneau JL, Massoubre C, Cabanes M, Lustenberger P (1992) Simple and sensitive determination of urea in serum and urine. ClinChem 38: 619-623.
- De Decker K, Cordonnier J, Jacobs W, Coucke V, Schepens P, et al. (2008) Fatal intoxication due to tramadol alone: case report and review of the literature. Forensic SciInt 175: 79-82.
- Shah NH, Thomas E, Jose R, Peedicayil J (2013) Tramadol inhibits the contractility of isolated human myometrium. Auton Autacoid Pharmacol 33: 1-5.
- Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, et al. (2005) Liver and kidney toxicity in chronic use of opioids: an experimental long term treatment model. J Biosci 30: 245-252.
- ZuhtuUtku S, Hakan D, Fazli E (2006) Histopathologic changes in liver induced by morphine and tramadol. The Pain Clinic 18: 321-325.
- Zhou P, Yan L, Yong Z, Yu G, Dong H, et al. (2013) Effect of thienorphine on the isolated uterine strips from pregnant rats. Eur J Pharmacol 703: 83-90.
- El-Gaafarawi IM, Hassan GB, Fouad G (2013) Toxic effects of paroxetine on sexual and reproductive functions of rats. The Egyptian Journal of Hospital Medicine 21: 16-32.
- Gitnick G, Labrecque, Moody F (1992) Diseases of the liver and billiary tract. Mosby- year book
- Sebnem DW, Handerson AR (1999) Clinical Enzymology in: Burtis CA. and Ashwood FR. Editors. Tietz Textbook of clinical chemistry (3rd edition), W.B. Saunders Company, Philadelphia.
- Singhal PC, Sharma P, Sanwal V, Prasad A, Kapasi A, et al. (1998) Morphine modulates proliferation of kidney fibroblasts. Kidney Int 53: 350-357.
- Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, et al. (2005) Liver and kidney toxicity in chronic use of opioids: an experimental long term treatment model. J Biosci 30: 245-252.
- Wu WN, McKown LA, Gauthier AD, Jones WJ, Raffa RB (2001) Metabolism of the analgesic drug, tramadol hydrochloride, in rat and dog. Xenobiotica 31: 423-441.
- Caju FM, Gian, Queiroz GD, Sandra MT, Bruno MT, et al.(2012) Opioid system manipulation during testicular development: results on sperm production and sertoli cells population. ActaScientiarum Biological Sciences 33:219–225.
- Heidari Z, Mahmoudzadeh-Sagheb H, Kohan F (2012) A Quantitative and Qualitative Study of Rat Testis Following Administration of Methadone and Buprenorphine. Int J High Risk 1: 14-17.
- Abou El Fatoh MF, Farag M, Sayed AE, Kamel MA, Nora E. Abdel-Hamed, et al. (2014) Some biochemical, neurochemical, pharmacotoxicological and histopathological alterations induced by long-term administration of tramadol in male rats. Int J Pharm Sci 4: 565–571.
- Alvarez JG, Storey BT (1995) Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. MolReprodDev 42: 334-346.
- Yassa HA, DawoodAel W, Shehata MM, Abdel-Hady RH, Aal KM (2010) Subchronic toxicity of cannabis leaves on male albino rats. Hum ExpToxicol 29: 37-47.
- Tennese AA, Wevrick R (2011) Impaired hypothalamic regulation of endocrine function and delayed counterregulatory response to hypoglycemia in Magel2-null mice. Endocrinology 152: 967-978.
- Abdellatief RB, Elgamal DA, Mohamed EE (2014) Effects of chronic tramadol administration on testicular tissue in rats: an experimental study. Andrologia 47: 674-679.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences